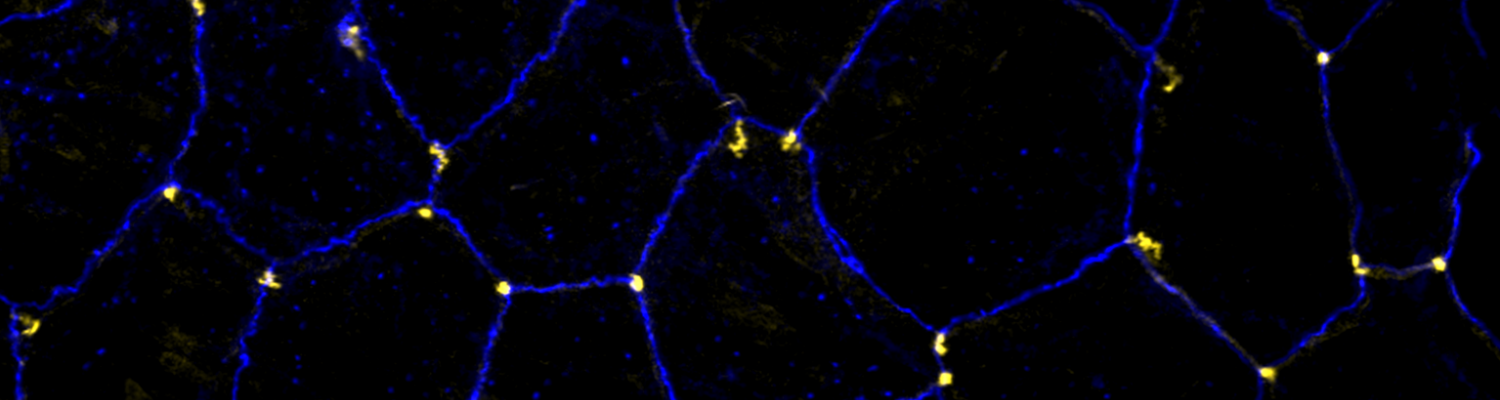
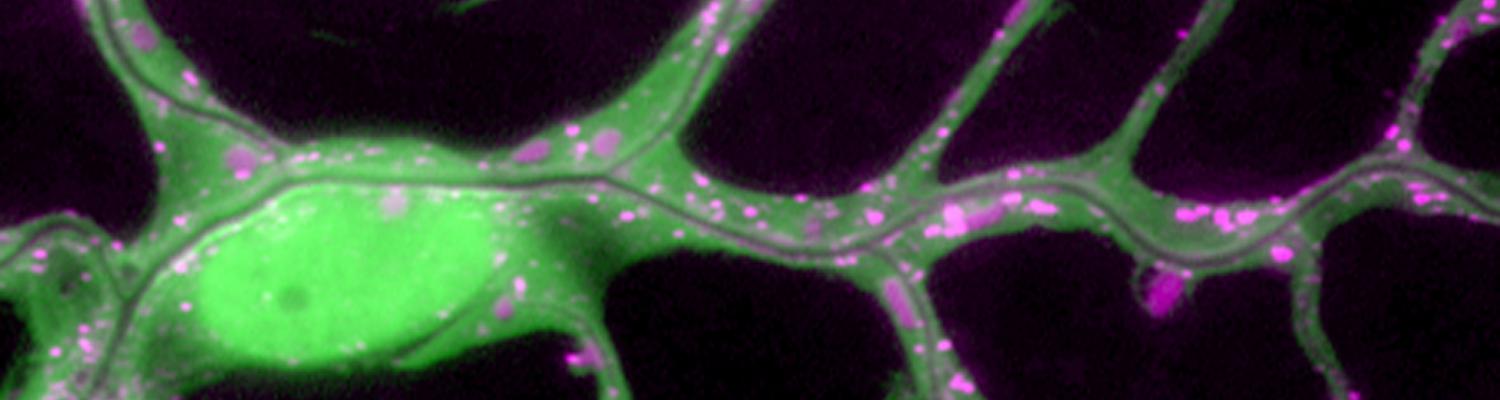
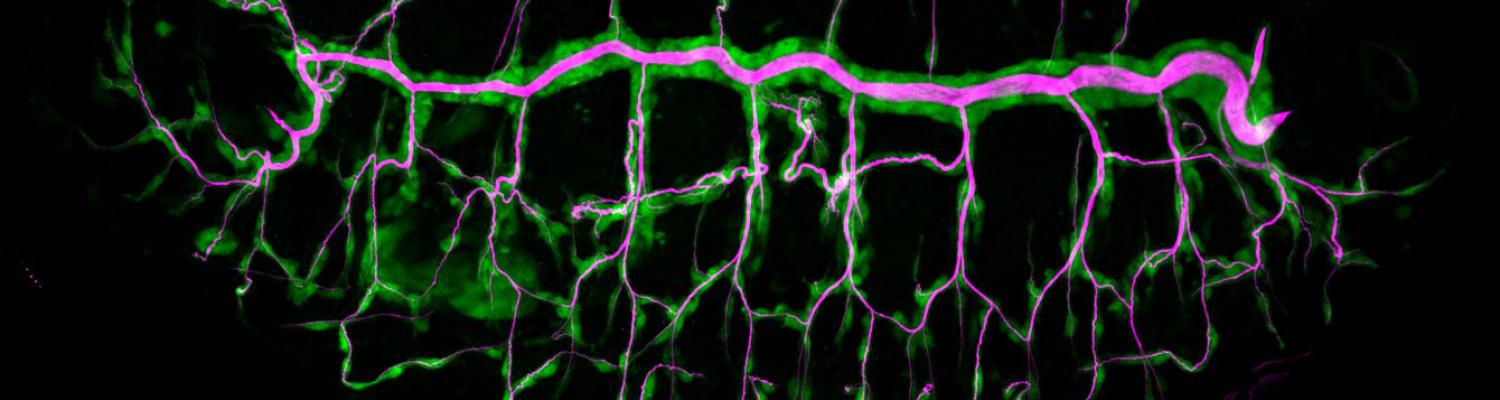
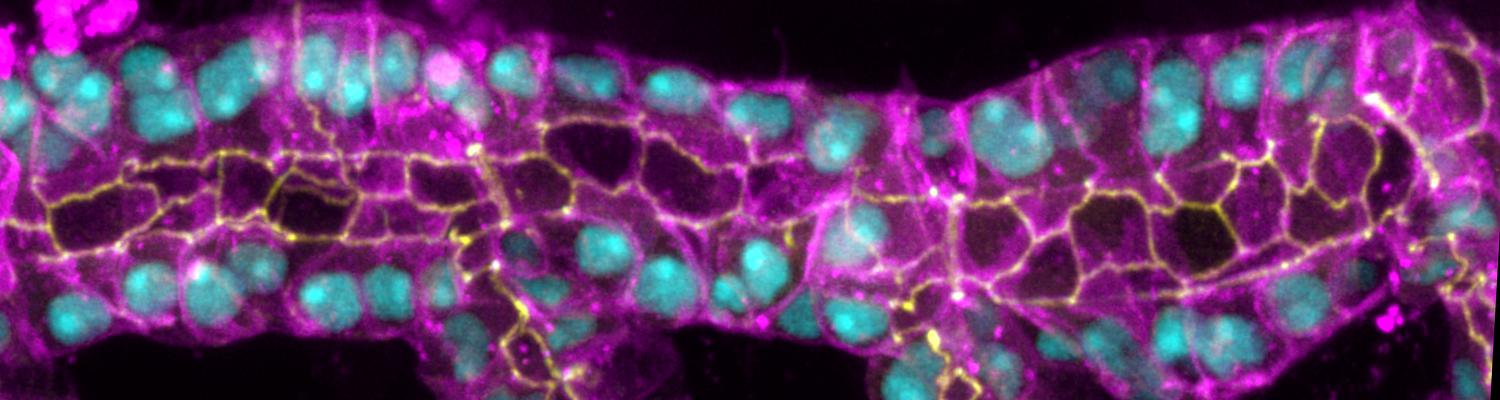
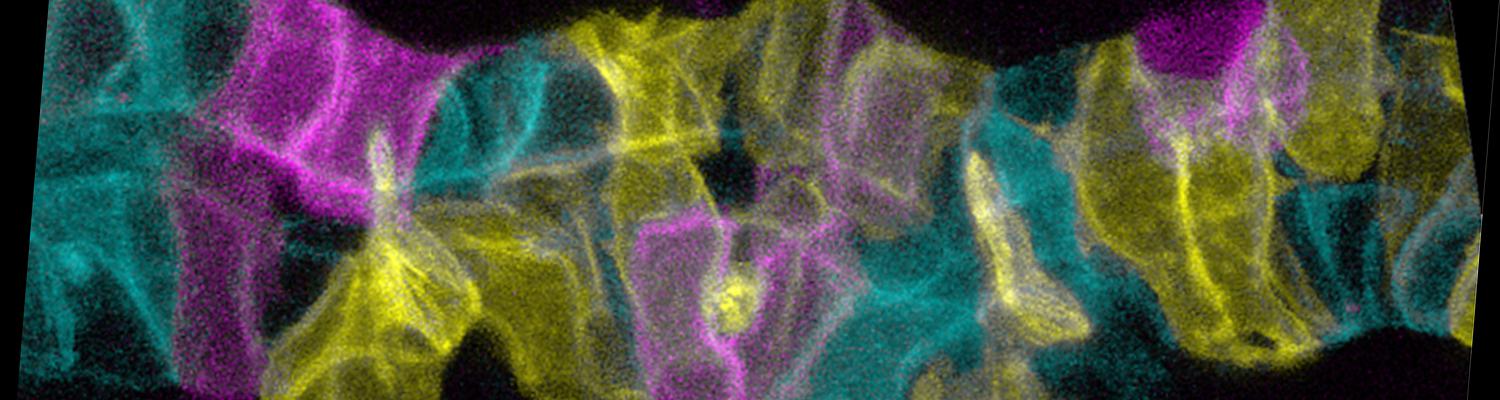
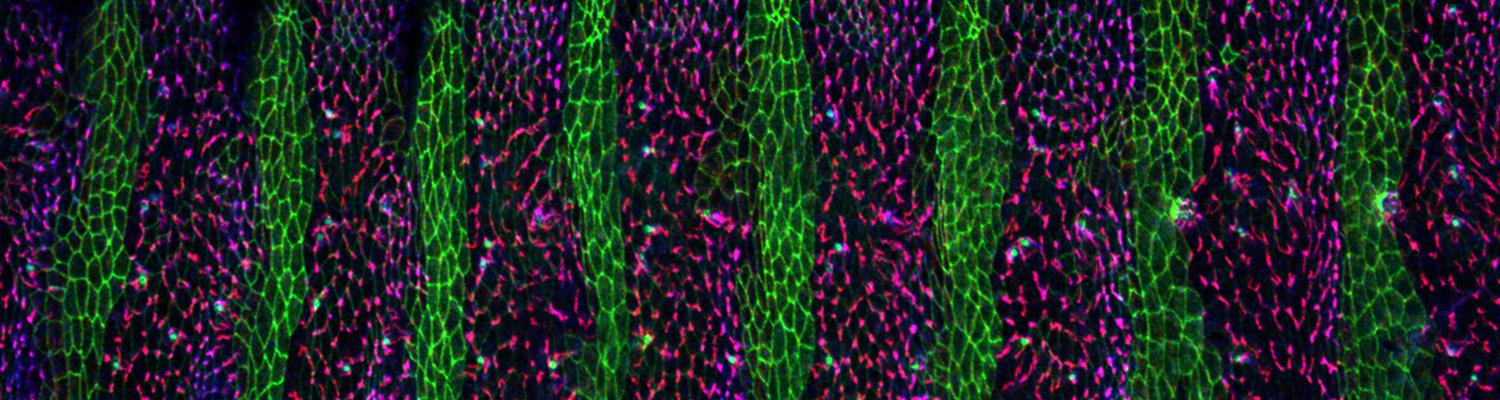
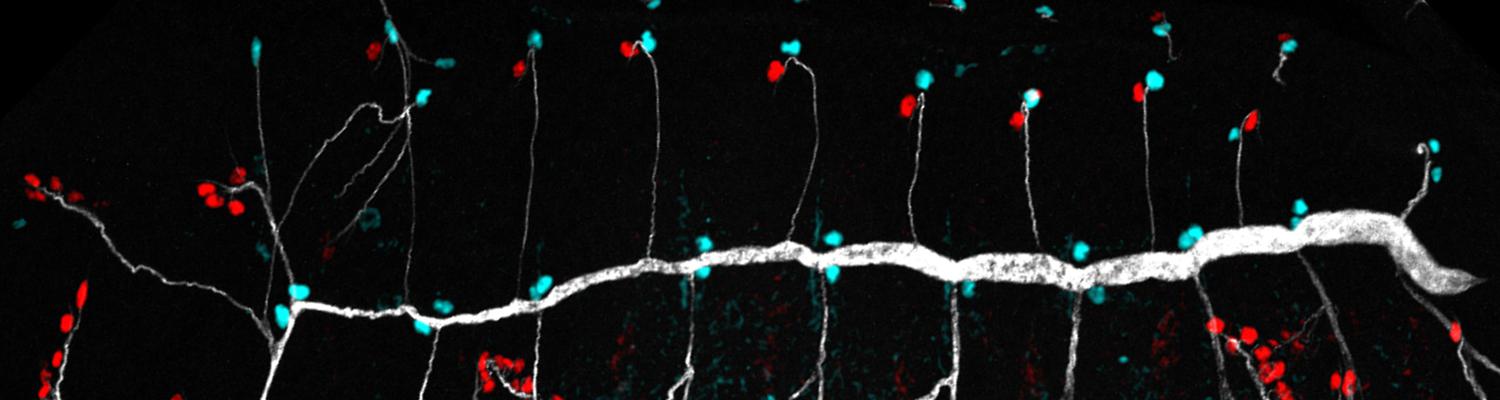
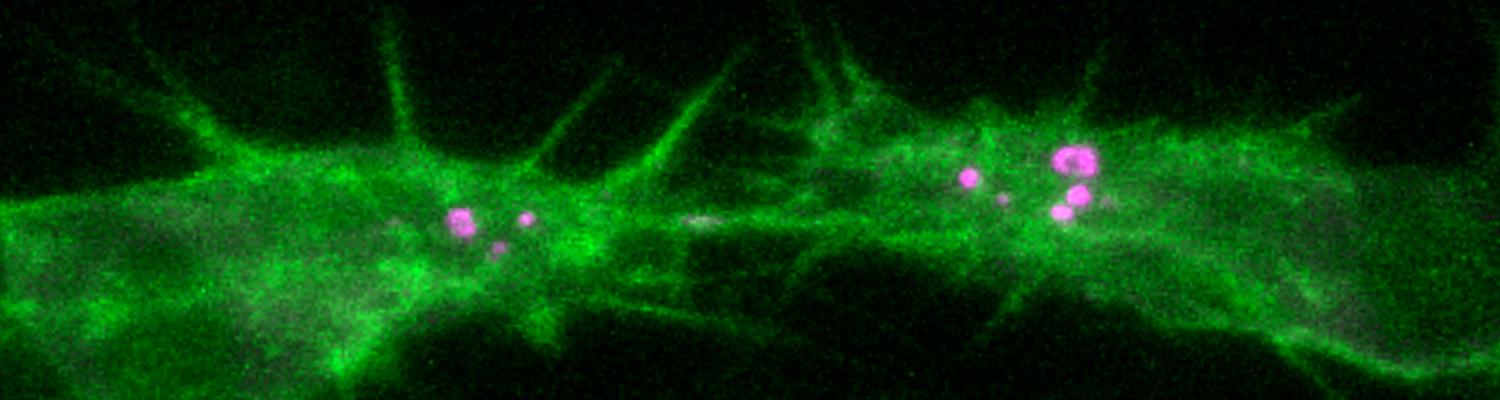
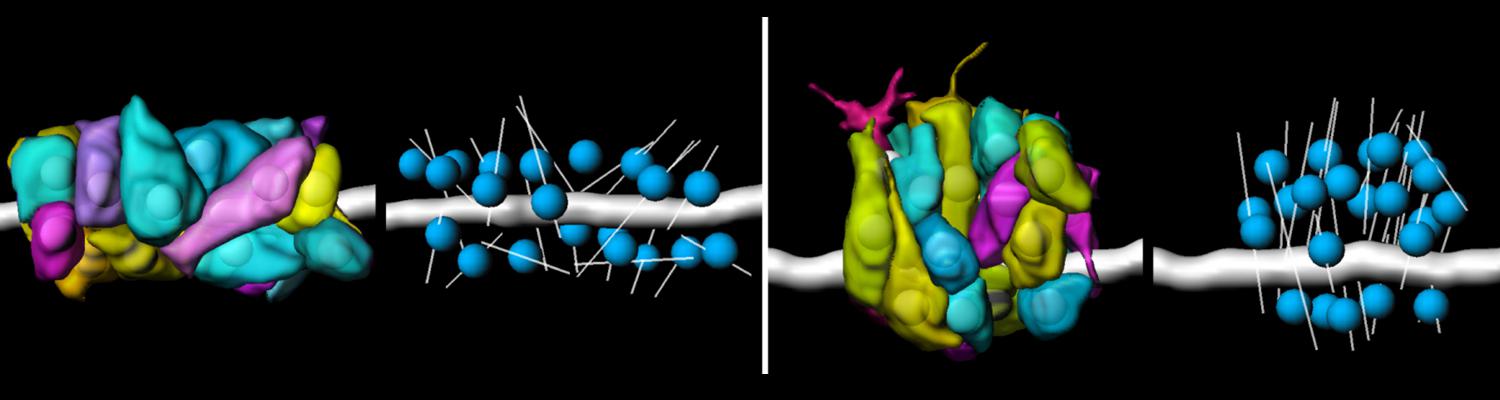
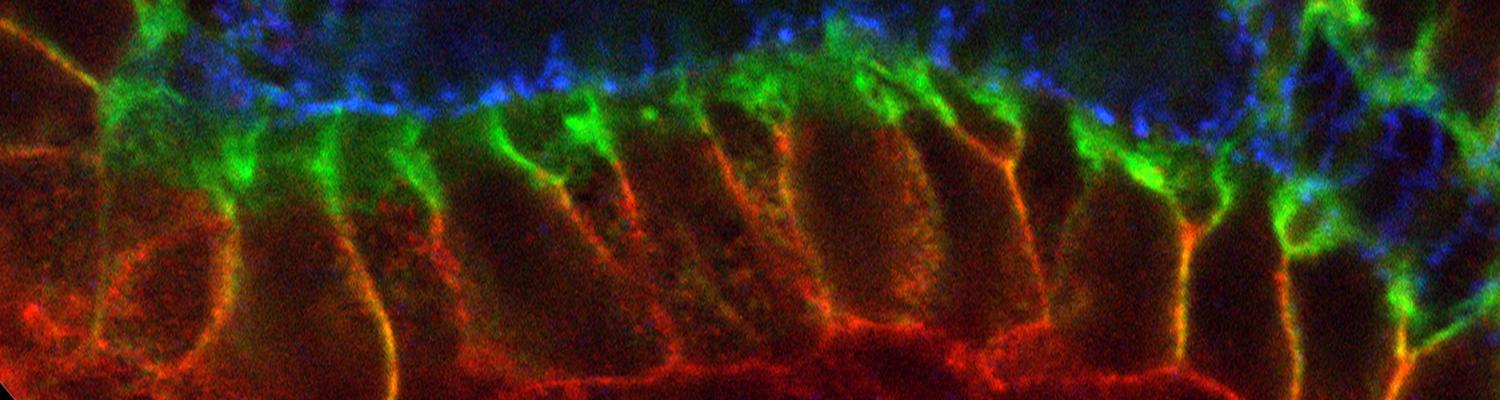
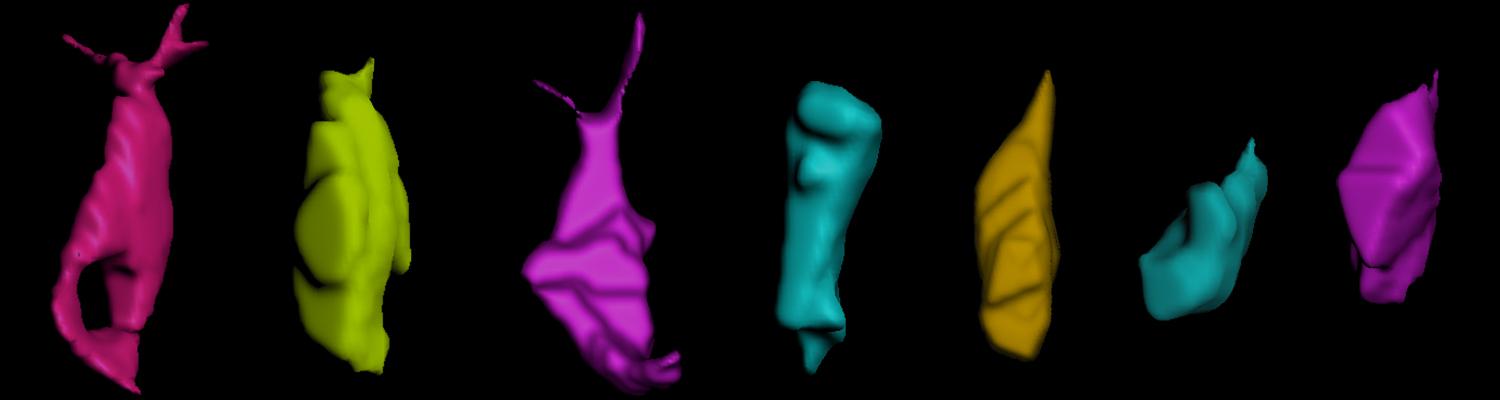
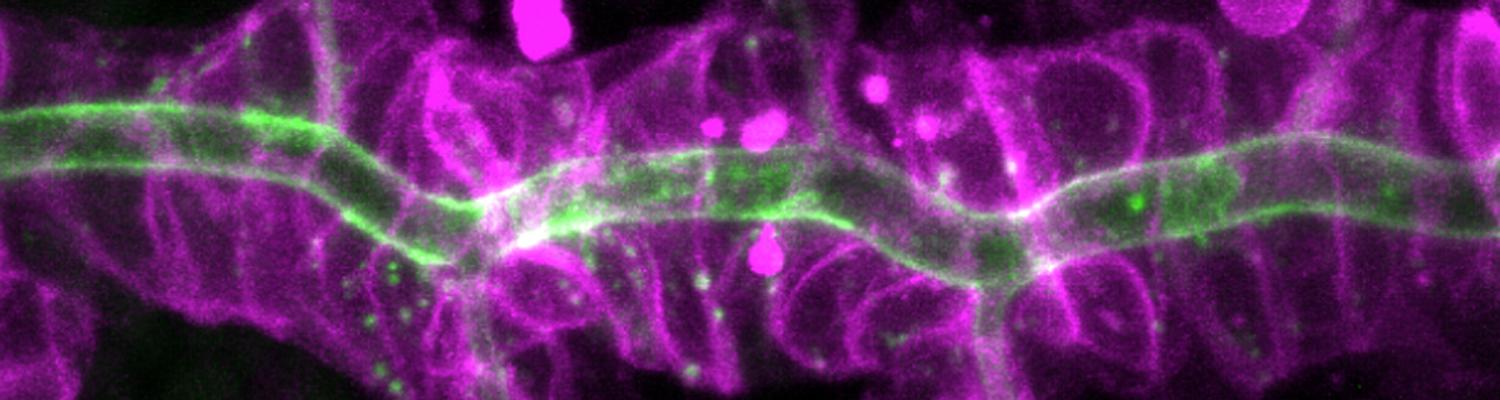
We study how tubular networks develop from initially separate units. In the vascular system separate endothelial sprouts fuse to build tubular bridges (anastomoses). Despite the fundamental role of this process in organogenesis and pathology, the mechanisms that govern epithelial tube fusion are not well understood. Tube fusion events resembling vascular anastomosis formation also occur during development of the tracheal system. Tube fusion involves directed migration of cells towards the fusion point, formation of a new cell-cell junction, and finally the connection of adjacent tubes. We aim to understand the mechanism of membrane fusion during the connection of tracheal tubes. We use in vivo cell labeling techniques combined with high-resolution light and electron microscopy to define the intermediates of the fusion process at the cellular and ultrastructural level. To identify new components of the underlying cellular machinery, we characterize fusion-defective mutants, which we isolated in genetic screens (Caviglia and Luschnig 20143. Answering basic questions about lumen formation and conversion of cellular topology in the Drosophila tracheal tube fusion model can provide a conceptual framework to help elucidate similar processes, such as vascular anastomosis and pronephric duct fusion, in more complex vertebrate systems.
Membrane Dynamics During Epithelial Tube Fusion
Related Publications
By: Caviglia S, Fischer EJ, Brankatschk M, Eaton S, Luschnig S
Nature Cell Biology | | Volume: 18 | Issue: 7 | 1-26 |Abstract
A crucial yet ill-defined step during the development of tubular networks, such as the vasculature, is the formation of connections (anastomoses) between pre-existing lumenized tubes. By studying tracheal tube anastomosis in Drosophila melanogaster, we uncovered a key role of secretory lysosome-related organelle (LRO) trafficking in lumen fusion. We identified the conserved calcium-binding protein Unc-13-4/Staccato (Stac) and the GTPase Rab39 as critical regulators of this process. Stac and Rab39 accumulate on dynamic vesicles, which form exclusively in fusion tip cells, move in a dynein-dependent manner, and contain late-endosomal, lysosomal, and SNARE components characteristic of LROs. The GTPase Arl3 is necessary and sufficient for Stac LRO formation and promotes Stac-dependent intracellular fusion of juxtaposed apical plasma membranes, thereby forming a transcellular lumen. Concomitantly, calcium is released locally from ER exit sites and apical membrane-associated calcium increases. We propose that calcium-dependent focused activation of LRO exocytosis restricts lumen fusion to appropriate domains within tip cells.
By: Caviglia S, Luschnig S
Seminars in Cell and Developmental Biology | | Volume: 31 | 82-90 |Abstract
Organs like the vertebrate vascular system and the insect tracheal system develop from separate primordia that undergo fusion events to form interconnected tubular networks. Although the correct pattern of tubular connections (anastomoses) in these organs is crucial for their normal function, the cellular and molecular mechanisms that govern tube fusion are only beginning to be understood. The process of tube fusion involves tip cell specification, cell-cell recognition and contact formation, self-avoidance, changes in cell shape and topology, lumen formation, and luminal membrane fusion. Significant insights into the underlying cellular machinery have been provided by genetic studies of tracheal tube fusion in Drosophila. Here, we summarize these findings and we highlight similarities and differences between tube fusion processes in the Drosophila tracheae and in the vertebrate vascular system. We integrate the findings from studies in vivo with the important mechanistic insights that have been gained from the analysis of tubulogenesis in cultured cells to propose a mechanistic model of tube fusion, aspects of which are likely to apply to diverse organs and organisms.