Breadcrumb
- Home
- Publications
Publications
Key Publications
Abstract
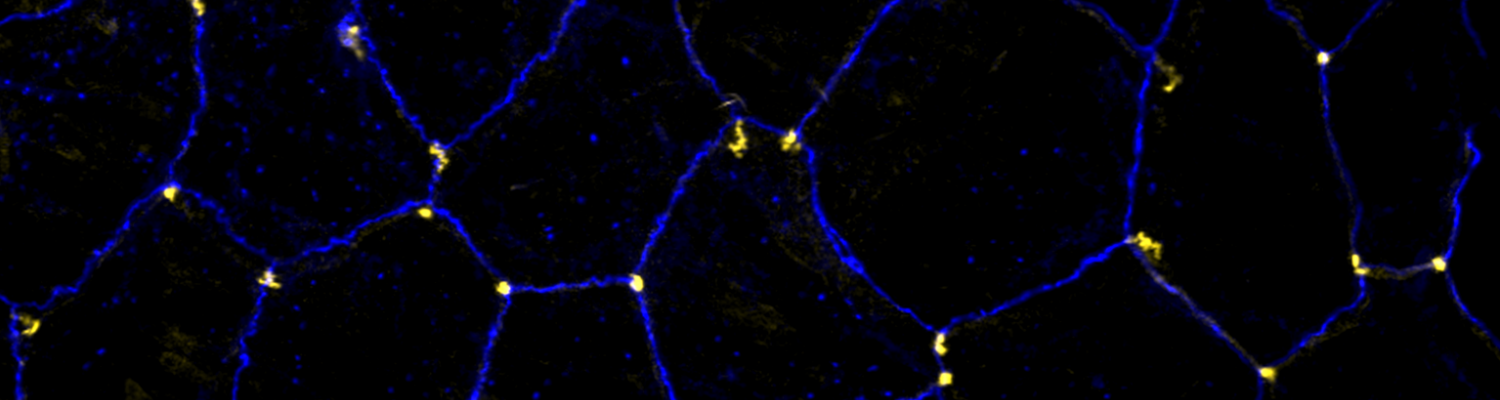
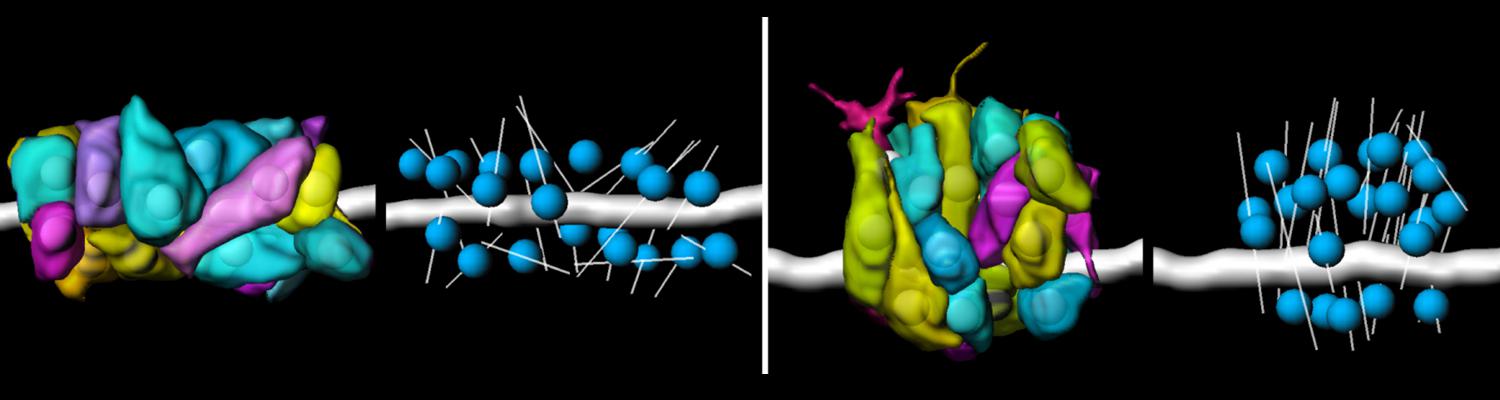
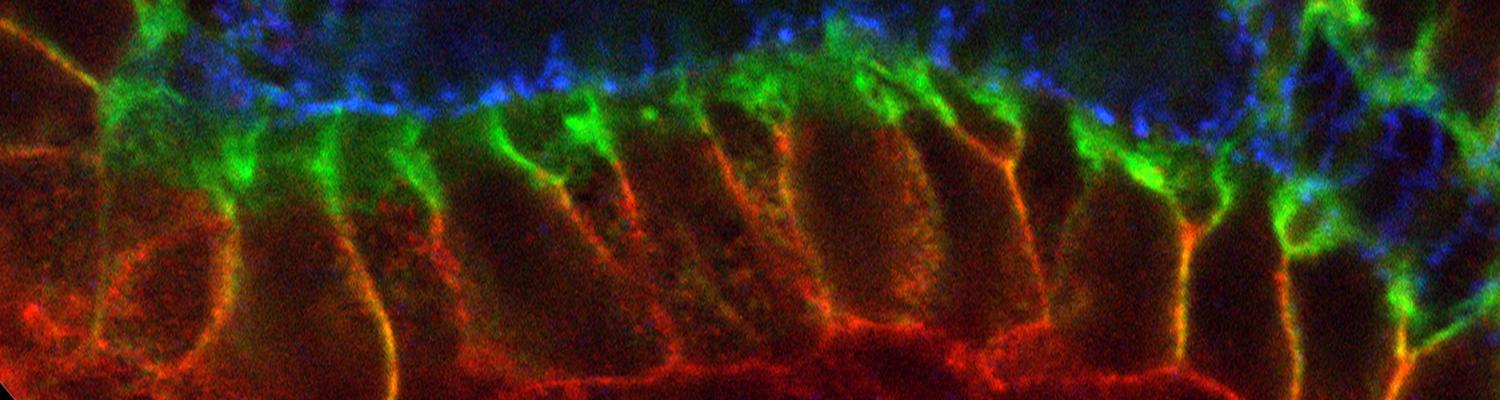
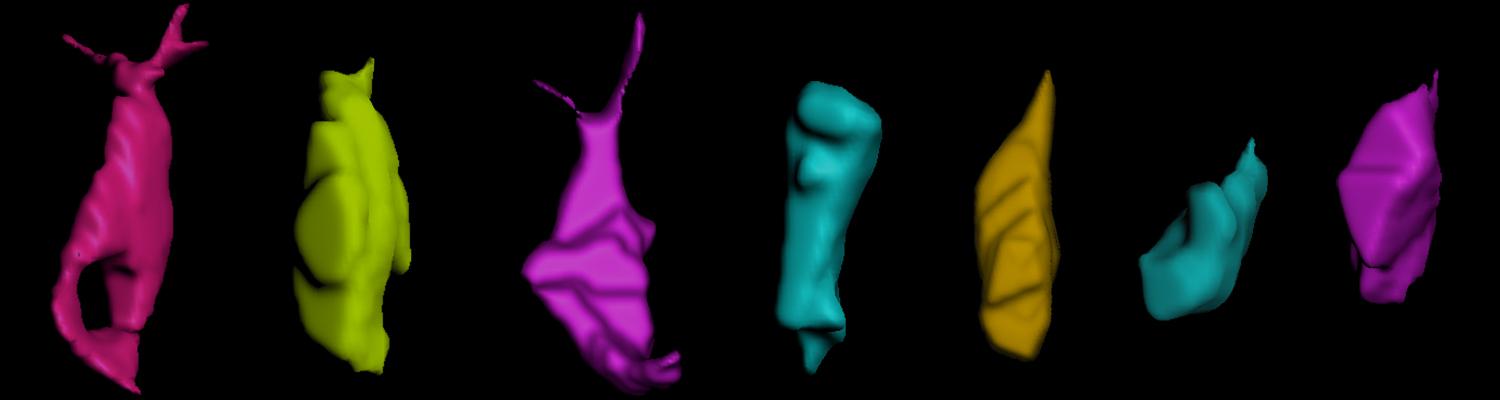
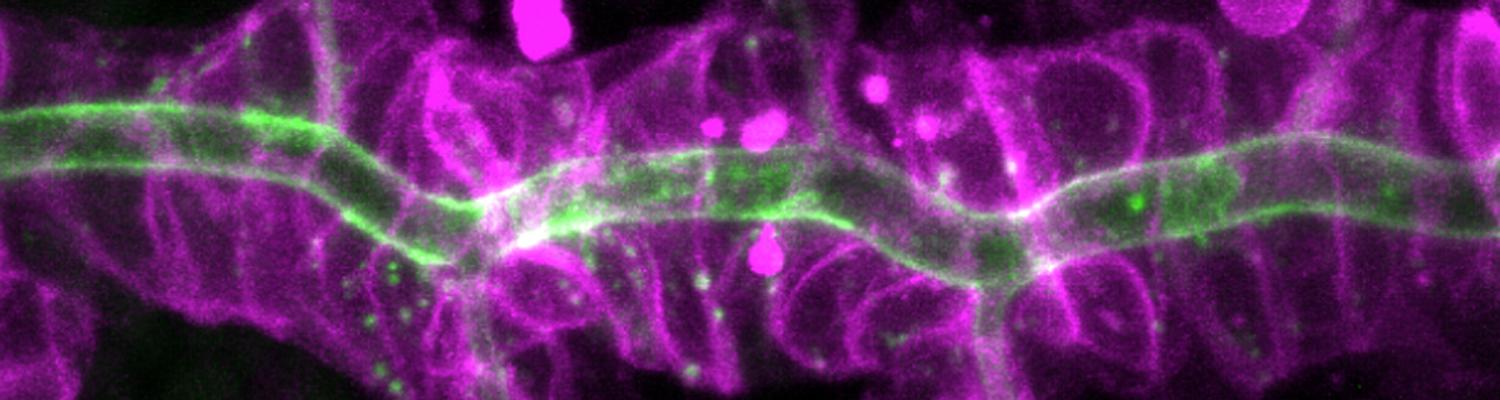
Transient opening of tricellular vertices controls paracellular transport through the follicle epithelium during Drosophila oogenesis
Paracellular permeability is regulated to allow solute transport or cell migration across epithelial or endothelial barriers. However, how cell-cell junction dynamics controls paracellular permeability is poorly understood. Here, we describe patency, a developmentally regulated process in Drosophila oogenesis, during which cell vertices in the follicular epithelium open transiently to allow paracellular transport of yolk proteins for uptake by the oocyte. We show that the sequential removal of E-cadherin, N-cadherin, NCAM/Fasciclin 2, and Sidekick from vertices precedes their basal-to-apical opening, while the subsequent assembly of tricellular occluding junctions marks the termination of patency and seals the paracellular barrier. E-cadherin-based adhesion is required to limit paracellular channel size, whereas stabilized adherens junctions, prolonged NCAM/Fasciclin 2 expression, blocked endocytosis, or increased actomyosin contractility prevent patency. Our findings reveal a key role of cell vertices as gateways controlling paracellular transport and demonstrate that dynamic regulation of adhesion and actomyosin contractility at vertices governs epithelial barrier properties.
| eLife | | pii: e48857 |
Abstract
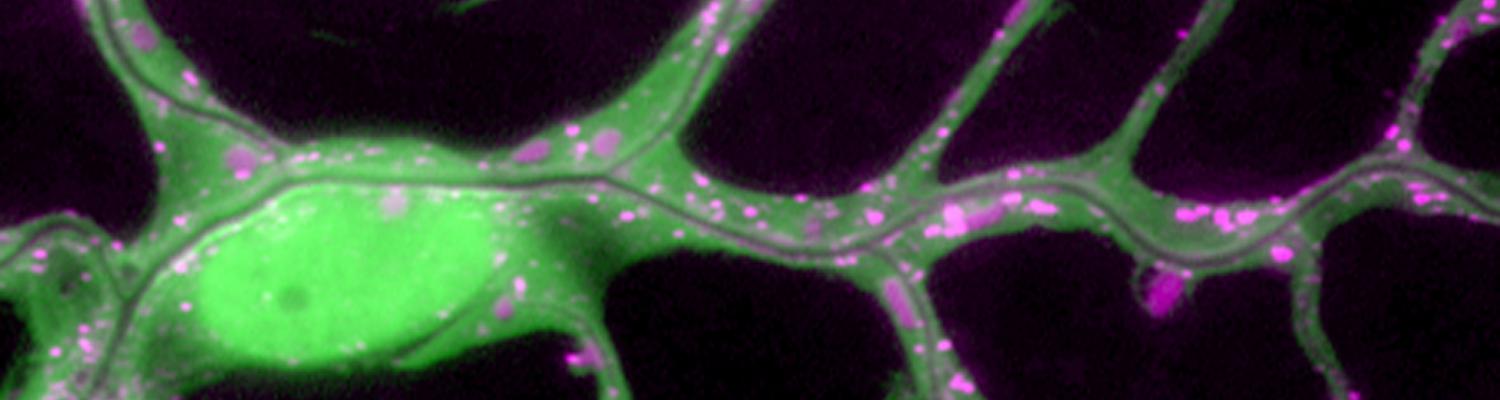
Matrix metalloproteinase 1 modulates invasive behavior of tracheal branches during entry into Drosophila flight muscles.
Tubular networks like the vasculature extend branches throughout animal bodies, but how developing vessels interact with and invade tissues is not well understood. We investigated the underlying mechanisms using the developing tracheal tube network of Drosophila indirect flight muscles (IFMs) as a model. Live imaging revealed that tracheal sprouts invade IFMs directionally with growth-cone-like structures at branch tips. Ramification inside IFMs proceeds until tracheal branches fill the myotube. However, individual tracheal cells occupy largely separate territories, possibly mediated by cell-cell repulsion. Matrix metalloproteinase 1 (MMP1) is required in tracheal cells for normal invasion speed and for the dynamic organization of growth-cone-like branch tips. MMP1 remodels the CollagenIV-containing matrix around branch tips, which show differential matrix composition with low CollagenIV levels, while Laminin is present along tracheal branches. Thus, tracheal-derived MMP1 sustains branch invasion by modulating the dynamic behavior of sprouting branches as well as properties of the surrounding matrix.
Cells experience different oxygen concentrations depending on location, organismal developmental stage, and physiological or pathological conditions. Responses to reduced oxygen levels (hypoxia) rely on the conserved Hypoxia-Inducible Factor 1 (HIF-1). Understanding the developmental and tissue-specific responses to changing oxygen levels has been limited by the lack of adequate tools for monitoring HIF-1 in vivo. To visualise and analyse HIF-1 dynamics in Drosophila, we used a hypoxia biosensor consisting of GFP fused to the oxygen-dependent degradation domain (ODD) of the HIF-1 homologue Sima. GFP-ODD responds to changing oxygen levels and to genetic manipulations of the hypoxia pathway, reflecting oxygen-dependent regulation of HIF-1 at the single-cell level. Ratiometric imaging of GFP-ODD and a red-fluorescent reference protein reveals tissue-specific differences in the cellular hypoxic status at ambient normoxia. Strikingly, cells in the larval brain show distinct hypoxic states that correlate with the distribution and relative densities of respiratory tubes. We present a set of genetic and image analysis tools that enable new approaches to map hypoxic microenvironments, to probe effects of perturbations on hypoxic signalling, and to identify new regulators of the hypoxia response.
Abstract
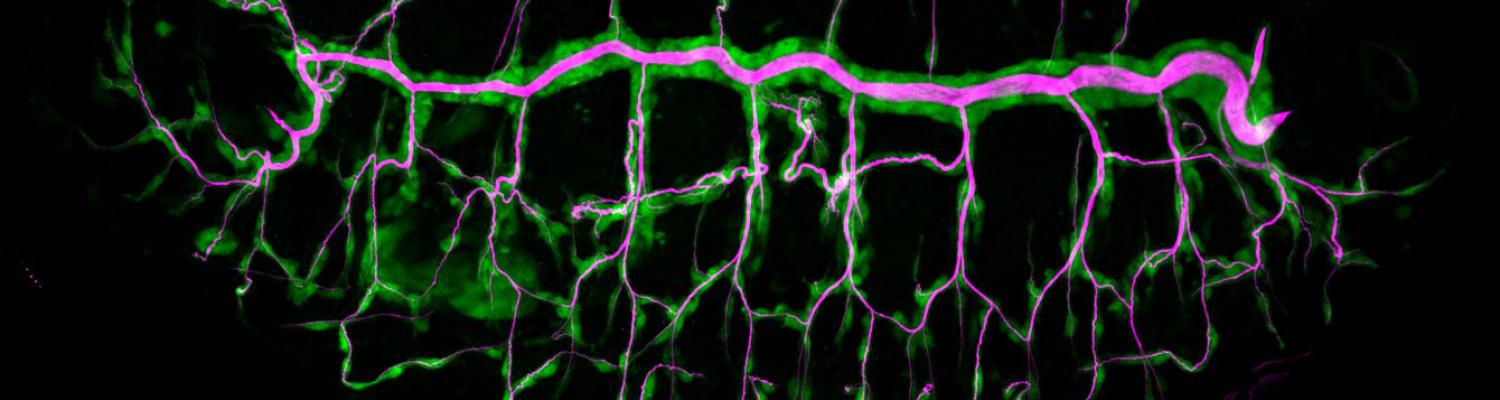
Staccato/Unc-13-4 controls secretory lysosome-mediated lumen fusion during epithelial tube anastomosis
A crucial yet ill-defined step during the development of tubular networks, such as the vasculature, is the formation of connections (anastomoses) between pre-existing lumenized tubes. By studying tracheal tube anastomosis in Drosophila melanogaster, we uncovered a key role of secretory lysosome-related organelle (LRO) trafficking in lumen fusion. We identified the conserved calcium-binding protein Unc-13-4/Staccato (Stac) and the GTPase Rab39 as critical regulators of this process. Stac and Rab39 accumulate on dynamic vesicles, which form exclusively in fusion tip cells, move in a dynein-dependent manner, and contain late-endosomal, lysosomal, and SNARE components characteristic of LROs. The GTPase Arl3 is necessary and sufficient for Stac LRO formation and promotes Stac-dependent intracellular fusion of juxtaposed apical plasma membranes, thereby forming a transcellular lumen. Concomitantly, calcium is released locally from ER exit sites and apical membrane-associated calcium increases. We propose that calcium-dependent focused activation of LRO exocytosis restricts lumen fusion to appropriate domains within tip cells.
Abstract
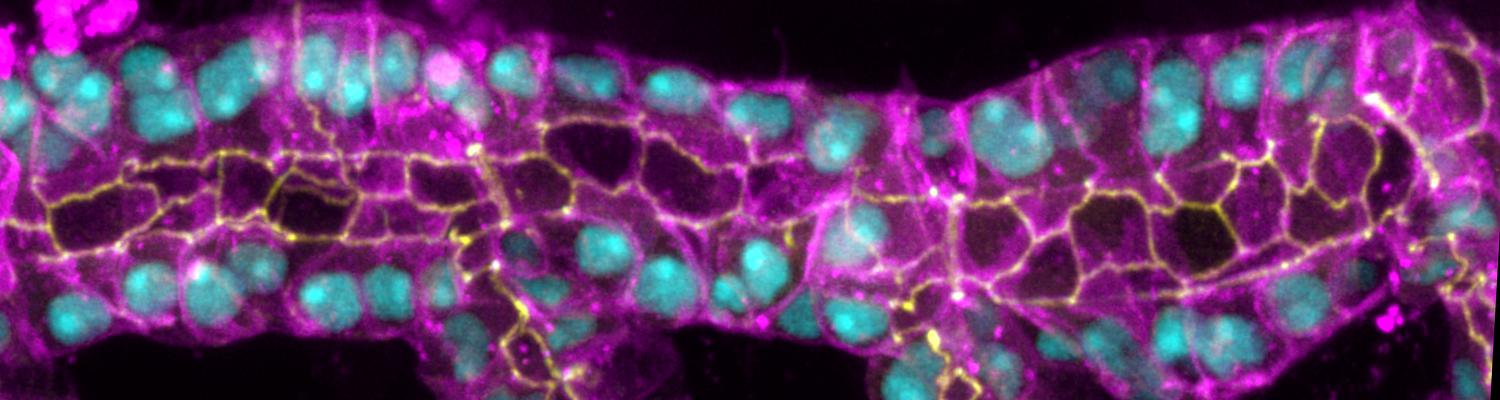
The triple-repeat protein Anakonda controls epithelial tricellular junction formation in Drosophila
In epithelia, specialized tricellular junctions (TCJs) mediate cell contacts at three-cell vertices. TCJs are fundamental to epithelial biology and disease, but only a few TCJ components are known, and how they assemble at tricellular vertices is not understood. Here we describe a transmembrane protein, Anakonda (Aka), which localizes to TCJs and is essential for the formation of tricellular, but not bicellular, junctions in Drosophila. Loss of Aka causes epithelial barrier defects associated with irregular TCJ structure and geometry, suggesting that Aka organizes cell corners. Aka is necessary and sufficient for accumulation of Gliotactin at TCJs, suggesting that Aka initiates TCJ assembly by recruiting other proteins to tricellular vertices. Aka's extracellular domain has an unusual tripartite repeat structure that may mediate self-assembly, directed by the geometry of tricellular vertices. Conversely, Aka's cytoplasmic tail is dispensable for TCJ localization. Thus, extracellular interactions, rather than TCJ-directed intracellular transport, appear to mediate TCJ assembly.
Abstract
Src42A-dependent polarized cell shape changes mediate epithelial tube elongation in Drosophila
Although many organ functions rely on epithelial tubes with correct dimensions, mechanisms underlying tube size control are poorly understood. We analyse the cellular mechanism of tracheal tube elongation in Drosophila, and describe an essential role of the conserved tyrosine kinase Src42A in this process. We show that Src42A is required for polarized cell shape changes and cell rearrangements that mediate tube elongation. In contrast, diametric expansion is controlled by apical secretion independently of Src42A. Constitutive activation of Src42A induces axial cell stretching and tracheal overelongation, indicating that Src42A acts instructively in this process. We propose that Src42A-dependent recycling of E-Cadherin at adherens junctions is limiting for cell shape changes and rearrangements in the axial dimension of the tube. Thus, we define distinct cellular processes that independently control axial and diametric expansion of a cylindrical epithelium in a developing organ. Whereas exocytosis-dependent membrane growth drives circumferential tube expansion, Src42A is required to orient membrane growth in the axial dimension of the tube.
All Publications
2022
Cell ablation is a key method in the research fields of developmental biology, tissue regeneration, and tissue homeostasis. Eliminating specific cell populations allows for characterizing interactions that control cell differentiation, death, behavior, and spatial organization of cells. Current methodologies for inducing cell death suffer from relatively slow kinetics, making them unsuitable for analyzing rapid events and following primary and immediate consequences of the ablation. To address this, we developed a cell-ablation system that is based on bacterial toxin/anti-toxin proteins and enables rapid and cell-autonomous elimination of specific cell types and organs in zebrafish embryos. A unique feature of this system is that it uses an anti-toxin, which allows for controlling the degree and timing of ablation and the resulting phenotypes. The transgenic zebrafish generated in this work represent a highly efficient tool for cell ablation, and this approach is applicable to other model organisms as demonstrated here for Drosophila.
Drosophila nephrocytes are an emerging model system for mammalian podocytes and proximal tubules as well as for the investigation of kidney diseases. Like podocytes, nephrocytes exhibit characteristics of epithelial cells, but the role of phospholipids in polarization of these cells is yet unclear. In epithelia, phosphatidylinositol(4,5)bisphosphate (PI(4,5)P2) and phosphatidylinositol(3,4,5)-trisphosphate (PI(3,4,5)P3) are asymmetrically distributed in the plasma membrane and determine apical-basal polarity. Here, we demonstrate that both phospholipids are present in the plasma membrane of nephrocytes, but only PI(4,5)P2 accumulates at slit diaphragms. Knockdown of Skittles, a phosphatidylinositol(4)phosphate 5-kinase, which produces PI(4,5)P2, abolished slit diaphragm formation and led to strongly reduced endocytosis. Notably, reduction in PI(3,4,5)P3 by overexpression of PTEN or expression of a dominant-negative phosphatidylinositol-3-kinase did not affect nephrocyte function, whereas enhanced formation of PI(3,4,5)P3 by constitutively active phosphatidylinositol-3-kinase resulted in strong slit diaphragm and endocytosis defects by ectopic activation of the Akt/mTOR pathway. Thus, PI(4,5)P2 but not PI(3,4,5)P3 is essential for slit diaphragm formation and nephrocyte function. However, PI(3,4,5)P3 has to be tightly controlled to ensure nephrocyte development.
Abstract
Tracheal tube fusion in Drosophila involves release of extracellular vesicles from multivesicular bodies.
Extracellular vesicles (EVs) comprise diverse types of cell-released membranous structures that are thought to play important roles in intercellular communication. While the formation and functions of EVs have been investigated extensively in cultured cells, studies of EVs in vivo have remained scarce. We report here that EVs are present in the developing lumen of tracheal tubes in Drosophila embryos. We define two distinct EV subpopulations, one of which contains the Munc13-4 (also known as UNC13D) homolog Staccato (Stac) and is spatially and temporally associated with tracheal tube fusion (anastomosis) events. The formation of Stac-positive luminal EVs depends on the tracheal tip-cell-specific GTPase Arl3 (also known as Dnd in Drosophila), which is also required for the formation of Stac-positive multivesicular bodies (MVBs), suggesting that Stac-positive EVs derive from fusion of Stac-positive MVBs with the luminal membrane in tip cells during anastomosis formation. The GTPases Rab27 and Rab35 cooperate downstream of Arl3 to promote Stac-positive MVB formation and tube fusion. We propose that Stac-positive MVBs act as membrane reservoirs that facilitate tracheal lumen fusion in a process regulated by Arl3, Rab27, Rab35 and Stac.
2021
Abstract
Transient opening of tricellular vertices controls paracellular transport through the follicle epithelium during Drosophila oogenesis
Paracellular permeability is regulated to allow solute transport or cell migration across epithelial or endothelial barriers. However, how cell-cell junction dynamics controls paracellular permeability is poorly understood. Here, we describe patency, a developmentally regulated process in Drosophila oogenesis, during which cell vertices in the follicular epithelium open transiently to allow paracellular transport of yolk proteins for uptake by the oocyte. We show that the sequential removal of E-cadherin, N-cadherin, NCAM/Fasciclin 2, and Sidekick from vertices precedes their basal-to-apical opening, while the subsequent assembly of tricellular occluding junctions marks the termination of patency and seals the paracellular barrier. E-cadherin-based adhesion is required to limit paracellular channel size, whereas stabilized adherens junctions, prolonged NCAM/Fasciclin 2 expression, blocked endocytosis, or increased actomyosin contractility prevent patency. Our findings reveal a key role of cell vertices as gateways controlling paracellular transport and demonstrate that dynamic regulation of adhesion and actomyosin contractility at vertices governs epithelial barrier properties.
2020
The biophysical and biochemical properties of live tissues are important in the context of development and disease. Methods for evaluating these properties typically involve destroying the tissue or require specialized technology and complicated analyses. Here, we present a novel, noninvasive methodology for determining the spatial distribution of tissue features within embryos, making use of nondirectionally migrating cells and software we termed “Landscape,” which performs automatized high-throughput three-dimensional image registration. Using the live migrating cells as bioprobes, we identified structures within the zebrafish embryo that affect the distribution of the cells and studied one such structure constituting a physical barrier, which, in turn, influences amoeboid cell polarity. Overall, this work provides a unique approach for detecting tissue properties without interfering with animal’s development. In addition, Landscape allows for integrating data from multiple samples, providing detailed and reliable quantitative evaluation of variable biological phenotypes in different organisms.
Abstract
Cells into tubes: Molecular and physical principles underlying lumen formation in tubular organs
Tubular networks, such as the vascular and respiratory systems, transport liquids and gases in multicellular organisms. The basic units of these organs are tubes formed by single or multiple cells enclosing a luminal cavity. The formation and maintenance of correctly sized and shaped lumina are fundamental steps in organogenesis and are essential for organismal homeostasis. Therefore, understanding how cells generate, shape and maintain lumina is crucial for understanding normal organogenesis as well as the basis of pathological conditions. Lumen formation involves polarized membrane trafficking, cytoskeletal dynamics, and the influence of intracellular as well as extracellular mechanical forces, such as cortical tension, luminal pressure or blood flow. Various tissue culture and in vivo model systems, ranging from MDCK cell spheroids to tubular organs in worms, flies, fish, and mice, have provided many insights into the molecular and cellular mechanisms underlying lumenogenesis and revealed key factors that regulate the size and shape of cellular tubes. Moreover, the development of new experimental and imaging approaches enabled quantitative analyses of intracellular dynamics and allowed to assess the roles of cellular and tissue mechanics during tubulogenesis. However, how intracellular processes are coordinated and regulated across scales of biological organization to generate properly sized and shaped tubes is only beginning to be understood. Here, we review recent insights into the molecular, cellular and physical mechanisms underlying lumen formation during organogenesis. We discuss how these mechanisms control lumen formation in various model systems, with a special focus on the morphogenesis of tubular organs in Drosophila.
2019
| eLife | pii: e48857 |
Abstract
Matrix metalloproteinase 1 modulates invasive behavior of tracheal branches during entry into Drosophila flight muscles.
Tubular networks like the vasculature extend branches throughout animal bodies, but how developing vessels interact with and invade tissues is not well understood. We investigated the underlying mechanisms using the developing tracheal tube network of Drosophila indirect flight muscles (IFMs) as a model. Live imaging revealed that tracheal sprouts invade IFMs directionally with growth-cone-like structures at branch tips. Ramification inside IFMs proceeds until tracheal branches fill the myotube. However, individual tracheal cells occupy largely separate territories, possibly mediated by cell-cell repulsion. Matrix metalloproteinase 1 (MMP1) is required in tracheal cells for normal invasion speed and for the dynamic organization of growth-cone-like branch tips. MMP1 remodels the CollagenIV-containing matrix around branch tips, which show differential matrix composition with low CollagenIV levels, while Laminin is present along tracheal branches. Thus, tracheal-derived MMP1 sustains branch invasion by modulating the dynamic behavior of sprouting branches as well as properties of the surrounding matrix.
2018
The nonsense-mediated mRNA decay (NMD) pathway is a cellular quality control and post-transcriptional gene regulatory mechanism and is essential for viability in most multicellular organisms. A complex of proteins has been identified to be required for NMD function to occur, however there is an incomplete understanding of the individual contributions of each of these factors to the NMD process. Central to the NMD process are three proteins, Upf1 (SMG-2), Upf2 (SMG-3), and Upf3 (SMG-4), which are found in all eukaryotes, with Upf1 and Upf2 being absolutely required for NMD in all organisms in which their functions have been examined. The other known NMD factors, Smg1, Smg5, Smg6, and Smg7 are more variable in their presence in different orders of organisms and are thought to have a more regulatory role. Here we present the first genetic analysis of the NMD factor Smg5 in Drosophila. Surprisingly, we find that unlike the other analyzed Smg genes in this organism, Smg5 is essential for NMD activity. We found this is due in part to a requirement for Smg5 in both the activity of Smg6-dependent endonucleolytic cleavage, as well as an additional Smg6-independent mechanism. Redundancy between these degradation pathways explains why some Drosophila NMD genes are not required for all NMD-pathway activity. We also found that while the NMD component Smg1 has only a minimal role in Drosophila NMD during normal conditions, it becomes essential when NMD activity is compromised by partial loss of Smg5 function. Our findings suggest that not all NMD complex components are required for NMD function at all times, but instead are utilized in a context dependent manner in vivo.
Abstract
Polarization-resolved microscopy reveals a muscle myosin motor-independent mechanism of molecular actin ordering during sarcomere maturation.
Sarcomeres are stereotyped force-producing mini-machines of striated muscles. Each sarcomere contains a pseudocrystalline order of bipolar actin and myosin filaments, which are linked by titin filaments. During muscle development, these three filament types need to assemble into long periodic chains of sarcomeres called myofibrils. Initially, myofibrils contain immature sarcomeres, which gradually mature into their pseudocrystalline order. Despite the general importance, our understanding of myofibril assembly and sarcomere maturation in vivo is limited, in large part because determining the molecular order of protein components during muscle development remains challenging. Here, we applied polarization-resolved microscopy to determine the molecular order of actin during myofibrillogenesis in vivo. This method revealed that, concomitantly with mechanical tension buildup in the myotube, molecular actin order increases, preceding the formation of immature sarcomeres. Mechanistically, both muscle and nonmuscle myosin contribute to this actin order gain during early stages of myofibril assembly. Actin order continues to increase while myofibrils and sarcomeres mature. Muscle myosin motor activity is required for the regular and coordinated assembly of long myofibrils but not for the high actin order buildup during sarcomere maturation. This suggests that, in muscle, other actin-binding proteins are sufficient to locally bundle or cross-link actin into highly regular arrays.
Abstract
The adherens junction-associated LIM domain protein Smallish regulates epithelial morphogenesis.
In epithelia, cells adhere to each other in a dynamic fashion, allowing the cells to change their shape and move along each other during morphogenesis. The regulation of adhesion occurs at the belt-shaped adherens junction, the zonula adherens (ZA). Formation of the ZA depends on components of the Par–atypical PKC (Par-aPKC) complex of polarity regulators. We have identified the Lin11, Isl-1, Mec-3 (LIM) protein Smallish (Smash), the orthologue of vertebrate LMO7, as a binding partner of Bazooka/Par-3 (Baz), a core component of the Par-aPKC complex. Smash also binds to Canoe/Afadin and the tyrosine kinase Src42A and localizes to the ZA in a planar polarized fashion. Animals lacking Smash show loss of planar cell polarity (PCP) in the embryonic epidermis and reduced cell bond tension, leading to severe defects during embryonic morphogenesis of epithelial tissues and organs. Overexpression of Smash causes apical constriction of epithelial cells. We propose that Smash is a key regulator of morphogenesis coordinating PCP and actomyosin contractility at the ZA.
2017
The organization of intracellular transport processes is adapted specifically to different cell types, developmental stages, and physiologic requirements. Some protein traffic routes are universal to all cells and constitutively active, while other routes are cell-type specific, transient, and induced under particular conditions only. Small GTPases of the Rab (Ras related in brain) subfamily are conserved across eukaryotes and regulate most intracellular transit pathways. The complete sets of Rab proteins have been identified in model organisms, and molecular principles underlying Rab functions have been uncovered. Rabs provide intracellular landmarks that define intracellular
transport sequences. Nevertheless, it remains a challenge to systematically map the subcellular
distribution of all Rabs and their functional interrelations. This task requires novel tools to precisely describe and manipulate the Rab machinery in vivo. Here we discuss recent findings about Rab roles during development and we consider novel approaches to investigate Rab functions in vivo.
Abstract
Faithful mRNA splicing depends on the Prp19 complex subunit faint sausage and is required for tracheal branching morphogenesis in Drosophila.
Morphogenesis requires the dynamic regulation of gene expression, including transcription, mRNA maturation and translation. Dysfunction of the general mRNA splicing machinery can cause surprisingly specific cellular phenotypes, but the basis for these effects is not clear. Here we show that the Drosophila faint sausage (fas) locus, implicated in epithelial morphogenesis and previously reported to encode a secreted immunoglobulin domain protein, in fact encodes a subunit of the spliceosome-activating Prp19 complex, which is essential for efficient pre-mRNA splicing. Loss of zygotic fas function globally impairs the efficiency of splicing, and is associated with widespread retention of introns in mRNAs and dramatic changes in gene expression. Surprisingly, despite these general effects, zygotic fas mutants show specific defects in tracheal cell migration during mid-embryogenesis when maternally supplied splicing factors have declined. We propose that tracheal branching, which relies on dynamic changes in gene expression, is particularly sensitive for efficient spliceosome function. Our results reveal an entry point to study requirements of the splicing machinery during organogenesis and provide a better understanding of disease phenotypes associated with mutations in general splicing factors.
2016
Cells experience different oxygen concentrations depending on location, organismal developmental stage, and physiological or pathological conditions. Responses to reduced oxygen levels (hypoxia) rely on the conserved Hypoxia-Inducible Factor 1 (HIF-1). Understanding the developmental and tissue-specific responses to changing oxygen levels has been limited by the lack of adequate tools for monitoring HIF-1 in vivo. To visualise and analyse HIF-1 dynamics in Drosophila, we used a hypoxia biosensor consisting of GFP fused to the oxygen-dependent degradation domain (ODD) of the HIF-1 homologue Sima. GFP-ODD responds to changing oxygen levels and to genetic manipulations of the hypoxia pathway, reflecting oxygen-dependent regulation of HIF-1 at the single-cell level. Ratiometric imaging of GFP-ODD and a red-fluorescent reference protein reveals tissue-specific differences in the cellular hypoxic status at ambient normoxia. Strikingly, cells in the larval brain show distinct hypoxic states that correlate with the distribution and relative densities of respiratory tubes. We present a set of genetic and image analysis tools that enable new approaches to map hypoxic microenvironments, to probe effects of perturbations on hypoxic signalling, and to identify new regulators of the hypoxia response.
Abstract
Staccato/Unc-13-4 controls secretory lysosome-mediated lumen fusion during epithelial tube anastomosis
A crucial yet ill-defined step during the development of tubular networks, such as the vasculature, is the formation of connections (anastomoses) between pre-existing lumenized tubes. By studying tracheal tube anastomosis in Drosophila melanogaster, we uncovered a key role of secretory lysosome-related organelle (LRO) trafficking in lumen fusion. We identified the conserved calcium-binding protein Unc-13-4/Staccato (Stac) and the GTPase Rab39 as critical regulators of this process. Stac and Rab39 accumulate on dynamic vesicles, which form exclusively in fusion tip cells, move in a dynein-dependent manner, and contain late-endosomal, lysosomal, and SNARE components characteristic of LROs. The GTPase Arl3 is necessary and sufficient for Stac LRO formation and promotes Stac-dependent intracellular fusion of juxtaposed apical plasma membranes, thereby forming a transcellular lumen. Concomitantly, calcium is released locally from ER exit sites and apical membrane-associated calcium increases. We propose that calcium-dependent focused activation of LRO exocytosis restricts lumen fusion to appropriate domains within tip cells.
Abstract
miR-190 enhances HIF-dependent responses to hypoxia in Drosophila by inhibiting the prolyl-4-hydroxylase Fatiga
Cellular and systemic responses to low oxygen levels are principally mediated by Hypoxia Inducible Factors (HIFs), a family of evolutionary conserved heterodimeric transcription factors, whose alpha- and beta-subunits belong to the bHLH-PAS family. In normoxia, HIFα is hydroxylated by specific prolyl-4-hydroxylases, targeting it for proteasomal degradation, while in hypoxia the activity of these hydroxylases decreases due to low oxygen availability, leading to HIFα accumulation and expression of HIF target genes. To identify microRNAs required for maximal HIF activity, we conducted an overexpression screen in Drosophila melanogaster, evaluating the induction of a HIF transcriptional reporter. miR-190 overexpression enhanced HIF-dependent biological responses, including terminal sprouting of the tracheal system, while in miR-190 loss of function embryos the hypoxic response was impaired. In hypoxic conditions, miR-190 expression was upregulated and required for induction of HIF target genes by directly inhibiting the HIF prolyl-4-hydroxylase Fatiga. Thus, miR-190 is a novel regulator of the hypoxia response that represses the oxygen sensor Fatiga, leading to HIFα stabilization and enhancement of hypoxic responses.
2015
Abstract
The triple-repeat protein Anakonda controls epithelial tricellular junction formation in Drosophila
In epithelia, specialized tricellular junctions (TCJs) mediate cell contacts at three-cell vertices. TCJs are fundamental to epithelial biology and disease, but only a few TCJ components are known, and how they assemble at tricellular vertices is not understood. Here we describe a transmembrane protein, Anakonda (Aka), which localizes to TCJs and is essential for the formation of tricellular, but not bicellular, junctions in Drosophila. Loss of Aka causes epithelial barrier defects associated with irregular TCJ structure and geometry, suggesting that Aka organizes cell corners. Aka is necessary and sufficient for accumulation of Gliotactin at TCJs, suggesting that Aka initiates TCJ assembly by recruiting other proteins to tricellular vertices. Aka's extracellular domain has an unusual tripartite repeat structure that may mediate self-assembly, directed by the geometry of tricellular vertices. Conversely, Aka's cytoplasmic tail is dispensable for TCJ localization. Thus, extracellular interactions, rather than TCJ-directed intracellular transport, appear to mediate TCJ assembly.
2014
Organs like the vertebrate vascular system and the insect tracheal system develop from separate primordia that undergo fusion events to form interconnected tubular networks. Although the correct pattern of tubular connections (anastomoses) in these organs is crucial for their normal function, the cellular and molecular mechanisms that govern tube fusion are only beginning to be understood. The process of tube fusion involves tip cell specification, cell-cell recognition and contact formation, self-avoidance, changes in cell shape and topology, lumen formation, and luminal membrane fusion. Significant insights into the underlying cellular machinery have been provided by genetic studies of tracheal tube fusion in Drosophila. Here, we summarize these findings and we highlight similarities and differences between tube fusion processes in the Drosophila tracheae and in the vertebrate vascular system. We integrate the findings from studies in vivo with the important mechanistic insights that have been gained from the analysis of tubulogenesis in cultured cells to propose a mechanistic model of tube fusion, aspects of which are likely to apply to diverse organs and organisms.
Abstract
The Macroglobulin complement-related transmembrane protein Mcr is essential for septate junction formation and epithelial barrier function in Drosophila
Occluding cell-cell junctions in epithelia form physical barriers that separate different membrane domains, restrict paracellular diffusion and prevent pathogens from spreading across tissues. In invertebrates, these functions are provided by septate junctions (SJs), the functional equivalent of vertebrate tight junctions. How the diverse functions of SJs are integrated and modulated in a multiprotein complex is not clear, and many SJ components are still unknown. Here we report the identification of Macroglobulin complement-related (Mcr), a member of the conserved α-2-macroglobulin (α2M) complement protein family, as a novel SJ-associated protein in Drosophila. Whereas α2M complement proteins are generally known as secreted factors that bind to surfaces of pathogens and target them for phagocytic uptake, Mcr represents an unusual α2M protein with a predicted transmembrane domain. We show that Mcr protein localizes to lateral membranes of epithelial cells, where its distribution overlaps with SJs. Several SJ components are required for the correct localization of Mcr. Conversely, Mcr is required in a cell-autonomous fashion for the correct membrane localization of SJ components, indicating that membrane-bound rather than secreted Mcr isoforms are involved in SJ formation. Finally, we show that loss of Mcr function leads to morphological, ultrastructural and epithelial barrier defects resembling mutants lacking SJ components. Our results, along with previous findings on the role of Mcr in phagocytosis, suggest that Mcr plays dual roles in epithelial barrier formation and innate immunity. Thus, Mcr represents a novel paradigm for investigating functional links between occluding junction formation and pathogen defense mechanisms.
Tubular epithelia come in various shapes and sizes to accommodate the specific needs for transport, excretion and absorption in multicellular organisms. The intestinal tract, glandular organs and conduits for liquids and gases are all lined by a continuous layer of epithelial cells, which form the boundary of the luminal space. Defects in epithelial architecture and lumen dimensions will impair transport and can lead to serious organ malfunctions. Not surprisingly, multiple cellular and molecular mechanisms contribute to the shape of tubular epithelial structures. One intriguing aspect of epithelial organ formation is the highly coordinate behavior of individual cells as they mold the mature lumen. Here, we focus on recent findings, primarily from Drosophila, demonstrating that informative cues can emanate from the developing organ lumen in the form of solid luminal material. The luminal material is produced by the surrounding epithelium and helps to coordinate changes in shape and arrangement of the very same cells, resulting in correct lumen dimensions.
2013
Abstract
The ETS domain transcriptional repressor Anterior open inhibits MAP Kinase and Wingless signaling to couple tracheal cell fate with branch identity
Cells at the tips of budding branches in the Drosophila tracheal system generate two morphologically different types of seamless tubes. Terminal cells (TCs) form branched lumenized extensions that mediate gas exchange at target tissues, whereas fusion cells (FCs) form ring-like connections between adjacent tracheal metameres. Each tracheal branch contains a specific set of TCs, FCs, or both, but the mechanisms that select between the two tip cell types in a branch-specific fashion are not clear. Here, we show that the ETS domain transcriptional repressor anterior open (aop) is dispensable for directed tracheal cell migration, but plays a key role in tracheal tip cell fate specification. Whereas aop globally inhibits TC and FC specification, MAPK signaling overcomes this inhibition by triggering degradation of Aop in tip cells. Loss of aop function causes excessive FC and TC specification, indicating that without Aop-mediated inhibition, all tracheal cells are competent to adopt a specialized fate. We demonstrate that Aop plays a dual role by inhibiting both MAPK and Wingless signaling, which induce TC and FC fate, respectively. In addition, the branch-specific choice between the two seamless tube types depends on the tracheal branch identity gene spalt major, which is sufficient to inhibit TC specification. Thus, a single repressor, Aop, integrates two different signals to couple tip cell fate selection with branch identity. The switch from a branching towards an anastomosing tip cell type may have evolved with the acquisition of a main tube that connects separate tracheal primordia to generate a tubular network.
2012
Abstract
Src42A-dependent polarized cell shape changes mediate epithelial tube elongation in Drosophila
Although many organ functions rely on epithelial tubes with correct dimensions, mechanisms underlying tube size control are poorly understood. We analyse the cellular mechanism of tracheal tube elongation in Drosophila, and describe an essential role of the conserved tyrosine kinase Src42A in this process. We show that Src42A is required for polarized cell shape changes and cell rearrangements that mediate tube elongation. In contrast, diametric expansion is controlled by apical secretion independently of Src42A. Constitutive activation of Src42A induces axial cell stretching and tracheal overelongation, indicating that Src42A acts instructively in this process. We propose that Src42A-dependent recycling of E-Cadherin at adherens junctions is limiting for cell shape changes and rearrangements in the axial dimension of the tube. Thus, we define distinct cellular processes that independently control axial and diametric expansion of a cylindrical epithelium in a developing organ. Whereas exocytosis-dependent membrane growth drives circumferential tube expansion, Src42A is required to orient membrane growth in the axial dimension of the tube.
Abstract
The Drosophila Sec7 domain Guanine Nucleotide Exchange Factor protein Gartenzwerg localizes at the cis-Golgi and is essential for epithelial tube expansion
Protein trafficking through the secretory pathway plays a key role in epithelial organ development and function. The expansion of tracheal tubes in Drosophila depends on trafficking of coatomer protein complex I (COPI)-coated vesicles between the Golgi complex and the endoplasmic reticulum (ER). However, it is not clear how this pathway is regulated. Here we describe an essential function of the Sec7 domain guanine nucleotide exchange factor (GEF) gartenzwerg (garz) in epithelial tube morphogenesis and protein secretion. garz is essential for the recruitment of COPI components and for normal Golgi organization. A GFP-Garz fusion protein is distributed in the cytoplasm and accumulates at the cis-Golgi. Localization to the Golgi requires the C-terminal part of Garz. Conversely, blocking the GDP-GTP nucleotide exchange reaction leads to constitutive Golgi localization, suggesting that Garz cycles in a GEF-activity-dependent manner between cytoplasmic and Golgi-membrane-localized pools. The related human ARF-GEF protein GBF1 can substitute for garz function in Drosophila tracheal cells, indicating that the relevant functions of these proteins are conserved. We show that garz interacts genetically with the ARF1 homolog ARF79F and with the ARF1-GAP homolog Gap69C, thus placing garz in a regulatory circuit that controls COPI trafficking in Drosophila. Interestingly, overexpression of garz causes accumulation of secreted proteins in the ER, suggesting that excessive garz activity leads to increased retrograde trafficking. Thus, garz might regulate epithelial tube morphogenesis and secretion by controlling the rate of trafficking of COPI vesicles.
2011
Abstract
Control of germline torso expression by the BTB/POZ domain protein Pipsqueak is required for embryonic terminal patterning in Drosophila
Early embryogenesis in Drosophila melanogaster is controlled by maternal gene products, which are deposited in the egg during oogenesis. It is not well understood how maternal gene expression is controlled during germline development. pipsqueak (psq) is a complex locus that encodes several nuclear protein variants containing a PSQ DNA-binding domain and a BTB/POZ domain. Psq proteins are thought to regulate germline gene expression through epigenetic silencing. While psq was originally identified as a posterior-group gene, we show here a novel role of psq in embryonic terminal patterning. We characterized a new psq loss-of-function allele, psq(rum), which specifically affects signaling by the Torso (Tor) receptor tyrosine kinase (RTK). Using genetic epistasis, gene expression analyses, and rescue experiments, we demonstrate that the sole function impaired by the psq(rum) mutation in the terminal system is an essential requirement for controlling transcription of the tor gene in the germline. In contrast, the expression of several other maternal genes, including those encoding Tor pathway components, is not affected by the mutation. Rescue of the psq(rum) terminal phenotype does not require the BTB/POZ domain, suggesting that the PSQ DNA-binding domain can function independently of the BTB/POZ domain. Our finding that tor expression is subject to dedicated transcriptional regulation suggests that different maternal genes may be regulated by multiple distinct mechanisms, rather than by a general program controlling nurse-cell transcription.
2010
Abstract
Localization and Activation of the Drosophila Protease Easter Requires the Saposin-Like ER Protein Seele
Drosophila embryonic dorsal-ventral polarity is generated by a series of serine protease processing events in the egg perivitelline space. Gastrulation Defective processes Snake, which then cleaves Easter, which then processes Spätzle into the activating ligand for the Toll receptor. seele was identified in a screen for mutations that, when homozygous in ovarian germline clones, lead to the formation of progeny embryos with altered embryonic patterning; maternal loss of seele function leads to the production of moderately dorsalized embryos. By combining constitutively active versions of Gastrulation Defective, Snake, Easter, and Spätzle with loss-of-function alleles of seele, we find that Seele activity is dispensable for Spätzle-mediated activation of Toll but is required for Easter, Snake, and Gastrulation Defective to exert their effects on dorsal-ventral patterning. Moreover, Seele function is required specifically for secretion of Easter from the developing embryo into the perivitelline space and for Easter processing. Seele protein resides in the endoplasmic reticulum of blastoderm embryos, suggesting a role in the trafficking of Easter to the perivitelline space, prerequisite to its processing and function. Easter transport to the perivitelline space represents a previously unappreciated control point in the signal transduction pathway that controls Drosophila embryonic dorsal-ventral polarity.
Abstract
Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila
Epithelial tubes in developing organs, such as mammalian lungs and insect tracheae, need to expand their initially narrow lumina to attain their final, functional dimensions. Despite its critical role for organ function, the cellular mechanism of tube expansion remains unclear. Tracheal tube expansion in Drosophila involves apical secretion and deposition of a luminal matrix, but the mechanistic role of secretion and the nature of forces involved in the process were not previously clear. Here we address the roles of cell-intrinsic and extrinsic processes in tracheal tube expansion. We identify mutations in the sec24 gene stenosis, encoding a cargo-binding subunit of the COPII complex. Via genetic-mosaic analyses, we show that stenosis-dependent secretion drives tube expansion in a cell-autonomous fashion. Strikingly, single cells autonomously adjust both tube diameter and length by implementing a sequence of events including apical membrane growth, cell flattening, and taenidial cuticle formation. Known luminal components are not required for this process. Thus, a cell-intrinsic program, rather than nonautonomous extrinsic cues, controls the dimensions of tracheal tubes. These results indicate a critical role of membrane-associated proteins in the process and imply a mechanism that coordinates autonomous behaviors of individual cells within epithelial structures.
2009
Polarity of many cell types is controlled by a protein complex consisting of Bazooka/PAR-3 (Baz), PAR-6 and atypical protein kinase C (aPKC). In Drosophila, the Baz-PAR-6-aPKC complex is required for the control of cell polarity in the follicular epithelium, in ectodermal epithelia and neuroblasts. aPKC is the main signaling component of this complex that functions by phosphorylating downstream targets, while the PDZ domain proteins Baz and PAR-6 control the subcellular localization and kinase activity of aPKC. We compared the mutant phenotypes of an aPKC null allele with those of four novel aPKC alleles harboring point mutations that abolish the kinase activity or the binding of aPKC to PAR-6. We show that these point alleles retain full functionality in the control of follicle cell polarity, but produce strong loss-of-function phenotypes in embryonic epithelia and neuroblasts. Our data, combined with molecular dynamics simulations, show that the kinase activity of aPKC and its ability to bind PAR-6 are only required for a subset of its functions during development, revealing tissue-specific differences in the way that aPKC controls cell polarity.
2008
Abstract
gammaCOP is required for apical protein secretion and epithelial morphogenesis in Drosophila melanogaster
BACKGROUND:
There is increasing evidence that tissue-specific modifications of basic cellular functions play an important role in development and disease. To identify the functions of COPI coatomer-mediated membrane trafficking in Drosophila development, we were aiming to create loss-of-function mutations in the gammaCOP gene, which encodes a subunit of the COPI coatomer complex.
PRINCIPAL FINDINGS:
We found that gammaCOP is essential for the viability of the Drosophila embryo. In the absence of zygotic gammaCOP activity, embryos die late in embryogenesis and display pronounced defects in morphogenesis of the embryonic epidermis and of tracheal tubes. The coordinated cell rearrangements and cell shape changes during tracheal tube morphogenesis critically depend on apical secretion of certain proteins. Investigation of tracheal morphogenesis in gammaCOP loss-of-function mutants revealed that several key proteins required for tracheal morphogenesis are not properly secreted into the apical lumen. As a consequence, gammaCOP mutants show defects in cell rearrangements during branch elongation, in tube dilation, as well as in tube fusion. We present genetic evidence that a specific subset of the tracheal defects in gammaCOP mutants is due to the reduced secretion of the Zona Pellucida protein Piopio. Thus, we identified a critical target protein of COPI-dependent secretion in epithelial tube morphogenesis.
CONCLUSIONS/SIGNIFICANCE:
These studies highlight the role of COPI coatomer-mediated vesicle trafficking in both general and tissue-specific secretion in a multicellular organism. Although COPI coatomer is generally required for protein secretion, we show that the phenotypic effect of gammaCOP mutations is surprisingly specific. Importantly, we attribute a distinct aspect of the gammaCOP phenotype to the effect on a specific key target protein.
Abstract
Wollknäuel is required for embryo patterning and encodes the Drosophila ALG5 UDP-glucose:dolichyl-phosphate glucosyltransferase
N-linked glycosylation is a prevalent protein modification in eukaryotic cells. Although glycosylation plays an important role in cell signaling during development, a role for N-linked glycosylation in embryonic patterning has not previously been described. In a screen for maternal factors involved in embryo patterning, we isolated mutations in Drosophila ALG5, a UDP-glucose:dolichyl-phosphate glucosyltransferase. Based on the embryonic cuticle phenotype, we designated the ALG5 locus wollknäuel (wol). Mutations in wol result in posterior segmentation phenotypes, reduced Dpp signaling, as well as impaired mesoderm invagination and germband elongation at gastrulation. The segmentation phenotype can be attributed to a post-transcriptional effect on expression of the transcription factor Caudal, whereas wol acts upstream of Dpp signalin by regulating dpp expression. The wol/ALG5 cDNA was able to partially complement the hypoglycosylation phenotype of alg5 mutant S. cerevisiae, whereas the two wol mutant alleles failed to complement. We show that reduced glycosylation in wol mutant embryos triggers endoplasmic reticulum stress and the unfolded protein response (UPR). As a result, phosphorylation of the translation factor eIF2alpha is increased. We propose a model in which translation of a few maternal mRNAs, including caudal, are particularly sensitive to increased eIF2alpha phosphorylation. According to this view, inappropriate UPR activation can cause specific patterning defects during embryo development.
Abstract
Pumilio Binds para mRNA and Requires Nanos and Brat to Regulate Sodium Current in Drosophila Motoneurons
Homeostatic regulation of ionic currents is of paramount importance during periods of synaptic growth or remodeling. Our previous work has identified the translational repressor Pumilio (Pum) as a regulator of sodium current (I(Na)) and excitability in Drosophila motoneurons. In this current study, we show that Pum is able to bind directly the mRNA encoding the Drosophila voltage-gated sodium channel paralytic (para). We identify a putative binding site for Pum in the 3' end of the para open reading frame (ORF). Characterization of the mechanism of action of Pum, using whole-cell patch clamp and real-time reverse transcription-PCR, reveals that the full-length protein is required for translational repression of para mRNA. Additionally, the cofactor Nanos is essential for Pum-dependent para repression, whereas the requirement for Brain Tumor (Brat) is cell type specific. Thus, Pum-dependent regulation of I(Na) in motoneurons requires both Nanos and Brat, whereas regulation in other neuronal types seemingly requires only Nanos but not Brat. We also show that Pum is able to reduce the level of nanos mRNA and as such identify a potential negative-feedback mechanism to protect neurons from overactivity of Pum. Finally, we show coupling between I(Na) (para) and I(K) (Shal) such that Pum-mediated change in para results in a compensatory change in Shal. The identification of para as a direct target of Pum represents the first ion channel to be translationally regulated by this repressor and the location of the binding motif is the first example in an ORF rather than in the canonical 3'-untranslated region of target transcripts.
2007
Abstract
Drosophila brakeless interacts with atrophin and is required for tailless-mediated transcriptional repression in early embryos
Complex gene expression patterns in animal development are generated by the interplay of transcriptional activators and repressors at cis-regulatory DNA modules (CRMs). How repressors work is not well understood, but often involves interactions with co-repressors. We isolated mutations in the brakeless gene in a screen for maternal factors affecting segmentation of the Drosophila embryo. Brakeless, also known as Scribbler, or Master of thickveins, is a nuclear protein of unknown function. In brakeless embryos, we noted an expanded expression pattern of the Krüppel (Kr) and knirps (kni) genes. We found that Tailless-mediated repression of kni expression is impaired in brakeless mutants. Tailless and Brakeless bind each other in vitro and interact genetically. Brakeless is recruited to the Kr and kni CRMs, and represses transcription when tethered to DNA. This suggests that Brakeless is a novel co-repressor. Orphan nuclear receptors of the Tailless type also interact with Atrophin co-repressors. We show that both Drosophila and human Brakeless and Atrophin interact in vitro, and propose that they act together as a co-repressor complex in many developmental contexts. We discuss the possibility that human Brakeless homologs may influence the toxicity of polyglutamine-expanded Atrophin-1, which causes the human neurodegenerative disease dentatorubral-pallidoluysian atrophy (DRPLA).
2006
Abstract
Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster
Genome-wide identification of RNAs associated with RNA-binding proteins is crucial for deciphering posttranscriptional regulatory systems. PUMILIO is a member of the evolutionary conserved Puf-family of RNA-binding proteins that repress gene expression posttranscriptionally. We generated transgenic flies expressing affinity-tagged PUMILIO under the control of an ovary-specific promoter, and we purified PUMILIO from whole adult flies and embryos and analyzed associated mRNAs by using DNA microarrays. Distinct sets comprising hundreds of mRNAs were associated with PUMILIO at the two developmental stages. Many of these mRNAs encode functionally related proteins, supporting a model for coordinated regulation of posttranscriptional modules by specific RNA-binding proteins. We identified a characteristic sequence motif in the 3′-untranslated regions of mRNAs associated with PUMILIO, and the sufficiency of this motif for interaction with PUMILIO was confirmed by RNA pull-down experiments with biotinylated synthetic RNAs. The RNA motif strikingly resembles the one previously identified for Puf3p, one of five Saccharomyces cerevisiae Puf proteins; however, proteins encoded by the associated mRNAs in yeast and Drosophila do not appear to be related. The results suggest extensive posttranscriptional regulation by PUMILIO and uncover evolutionary features of this conserved family of RNA-binding proteins.
Abstract
serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila
Many organs contain epithelial tubes that transport gases or liquids . Proper tube size and shape is crucial for organ function, but the mechanisms controlling tube diameter and length are poorly understood. Recent studies of tracheal (respiratory) tube morphogenesis in Drosophila show that chitin synthesis genes produce an expanding chitin cylinder in the apical (luminal) extracellular matrix (ECM) that coordinates the dilation of the surrounding epithelium . Here, we describe two genes involved in chitin modification, serpentine (serp) and vermiform (verm), mutations in which cause excessively long and tortuous tracheal tubes. The genes encode similar proteins with an LDL-receptor ligand binding motif and chitin binding and deacetylation domains. Both proteins are expressed and secreted during tube expansion and localize throughout the lumen in a chitin-dependent manner. Unlike previously characterized chitin pathway genes, serp and verm are not required for chitin synthesis or secretion but rather for its normal fibrillar structure. The mutations also affect structural properties of another chitinous matrix, epidermal cuticle. Our work demonstrates that chitin and the matrix proteins Serp and Verm limit tube elongation, and it suggests that tube length is controlled independently of diameter by modulating physical properties of the chitin ECM, presumably by N-deacetylation of chitin and conversion to chitosan.
2005
Many organs are composed of branched networks of epithelial tubes that transport vital fluids or gases. The proper size and shape of tubes are crucial for their transport function, but the molecular processes that govern tube size and shape are not well understood. Here we show that three genes required for tracheal tube morphogenesis in Drosophila melanogaster encode proteins involved in the synthesis and accumulation of chitin, a polymer of N-acetyl-beta-D-glucosamine that serves as a scaffold in the rigid extracellular matrix of insect cuticle. In all three mutants, developing tracheal tubes bud and extend normally, but the epithelial walls of the tubes do not expand uniformly, and the resultant tubes are grossly misshapen, with constricted and distended regions all along their lengths. The genes are expressed in tracheal cells during the expansion process, and chitin accumulates in the lumen of tubes, forming an expanding cylinder that we propose coordinates the behavior of the surrounding tracheal cells and stabilizes the expanding epithelium. These findings show that chitin regulates epithelial tube morphogenesis, in addition to its classical role protecting mature epithelia.
2004
Abstract
Drosophila p24 homologues eclair and baiser are necessary for the activity of the maternally expressed Tkv receptor during early embryogenesis
p24 proteins are assumed to play an important role in the transport of secreted and transmembrane proteins into membranes. However, only few cargo proteins are known that partially, but in no case completely require p24 proteins for membrane transport. Here, we show that two p24 proteins are essential for dorsoventral patterning of Drosophila melanogaster embryo. Mutations in the genes, eclair (eca) and baiser (bai), encoding two p24 proteins reduce signalling by the TGF-beta homologue, Dpp, in early embryos. This effect is strictly maternal and specific to early embryogenesis, as Dpp signalling in other contexts is not notably affected. We provide genetic evidence that in the absence of eca or bai function in the oocyte, the maternally expressed type I TGF-beta receptor Tkv is not active. We propose that during early embryogenesis eca and bai are specifically required for the activity of the maternal Tkv, while the zygotic Tkv is not affected in the mutant embryos. Mutations in either eca or bai are sufficient for the depletion of Tkv activity and no enhancement of the phenotypes was observed in embryos derived from oocytes mutant for both genes. The dependence of maternal Tkv protein on the products of p24 genes may serve as an in vivo model for studying p24 proteins.
Abstract
An F1 genetic screen for maternal-effect mutations affecting embryonic pattern formation in Drosophila melanogaster
Large-scale screens for female-sterile mutations have revealed genes required maternally for establishment of the body axes in the Drosophila embryo. Although it is likely that the majority of components involved in axis formation have been identified by this approach, certain genes have escaped detection. This may be due to (1) incomplete saturation of the screens for female-sterile mutations and (2) genes with essential functions in zygotic development that mutate to lethality, precluding their identification as female-sterile mutations. To overcome these limitations, we performed a genetic mosaic screen aimed at identifying new maternal genes required for early embryonic patterning, including zygotically required ones. Using the Flp-FRT technique and a visible germline clone marker, we developed a system that allows efficient screening for maternal-effect phenotypes after only one generation of breeding, rather than after the three generations required for classic female-sterile screens. We identified 232 mutants showing various defects in embryonic pattern or morphogenesis. The mutants were ordered into 10 different phenotypic classes. A total of 174 mutants were assigned to 86 complementation groups with two alleles on average. Mutations in 45 complementation groups represent most previously known maternal genes, while 41 complementation groups represent new loci, including several involved in dorsoventral, anterior-posterior, and terminal patterning.
2003
Many organs including the mammalian lung and vascular system consist of branched tubular networks that transport essential gases or fluids, but the genetic programs that control the development of these complex three-dimensional structures are not well understood. The Drosophila melanogaster tracheal (respiratory) system is a network of interconnected epithelial tubes that transports oxygen and other gases in the body and provides a paradigm of branching morphogenesis. It develops by sequential sprouting of primary, secondary, and terminal branches from an epithelial sac of approximately 80 cells in each body segment of the embryo. Mapping of the cell movements and shape changes during the sprouting process has revealed that distinct mechanisms of epithelial migration and tube formation are used at each stage of branching. Genetic dissection of the process has identified a general program in which a fibroblast growth factor (FGF) and fibroblast growth factor receptor (FGFR) are used repeatedly to control branch budding and outgrowth. At each stage of branching, the mechanisms controlling FGF expression and the downstream signal transduction pathway change, altering the pattern and structure of the branches that form. During terminal branching, FGF expression is regulated by hypoxia, ensuring that tracheal structure matches cellular oxygen need. A branch diversification program operates in parallel to the general budding program: Regional signals locally modify the general program, conferring specific structural features and other properties on individual branches, such as their substrate outgrowth preferences, differences in tube size and shape, and the ability to fuse to other branches to interconnect the network.
Abstract
Krapfen/dMyd88 is required for the establishment of dorsoventral pattern in the Drosophila embryo
In Drosophila, the dorsoventral axis is set up by the action of the dorsal group of genes and cactus, which have been ordered genetically in a linear pathway. We have identified and characterised krapfen (kra) as a new member of the dorsal-group genes. kra encodes for the Drosophila homologue of MyD88, an adapter protein operating in the mammalian IL-1 pathway. Epistasis experiments reveal that kra acts between the receptor Toll and the cytoplasmic factor Tube. We show that there is a direct interaction between Kra and Tube presumably mediated by the death domains present in both proteins. Tube in turn interacts with its downstream effector Pelle through death domain association. We therefore suggest that upon Toll activation, Kra associates with Pelle and Tube, in an heterotrimeric complex.
2002
Abstract
Gamma-tubulin37C and gamma-tubulin ring complex protein 75 are essential for bicoid RNA localization during Drosophila oogenesis
bicoid (bcd) RNA localization requires the activity of exuperantia and swallow at sequential steps of oogenesis and is microtubule dependent. In a genetic screen, we identified two novel genes essential for bcd RNA localization. They encode maternal gamma-Tubulin37C (gammaTub37C) and gamma-tubulin ring complex protein 75 (Dgrip75), both of which are gamma-tubulin ring complex components. Mutations in these genes specifically affect bcd RNA localization, whereas other microtubule-dependent processes during oogenesis are not impaired. This provides direct evidence that a subset of microtubules organized by the gamma-tubulin ring complex is essential for localization of bcd RNA. At stage 10b, we find gammaTub37C and Dgrip75 anteriorly concentrated and propose the formation of a microtubule-organizing center at the anterior pole of the oocyte.
2000
Abstract
The Drosophila SHC adaptor protein is required for signaling by a subset of receptor tyrosine kinases
Receptor tyrosine kinases (RTKs) transduce signals via cytoplasmic adaptor proteins to downstream signaling components. We have identified loss-of-function mutations in dshc, the Drosophila homolog of the mammalian adaptor protein SHC. A point mutation in the phosphotyrosine binding (PTB) domain completely abolishes DSHC function and provides in vivo evidence for the function of PTB domains. Unlike other adaptor proteins, DSHC is involved in signaling by only a subset of RTKs: dshc mutants show defects in Torso and DER but not Sevenless signaling, which is confirmed by epistasis experiments. We show by double-mutant analysis that the adaptors DOS, DRK, and DSHC act in parallel to transduce the Torso signal. Our results suggest that DSHC confers specificity to receptor signaling.