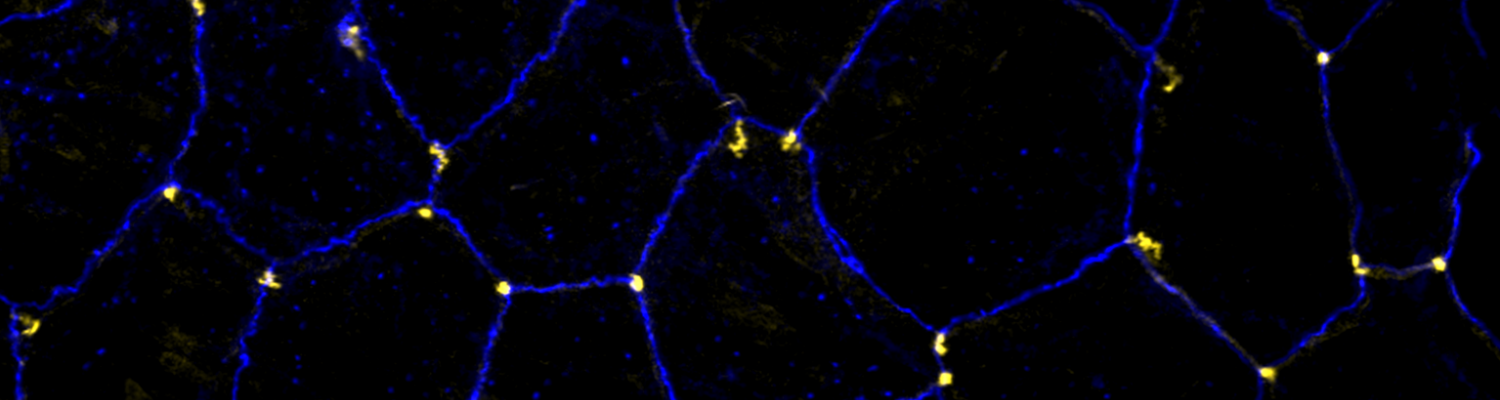
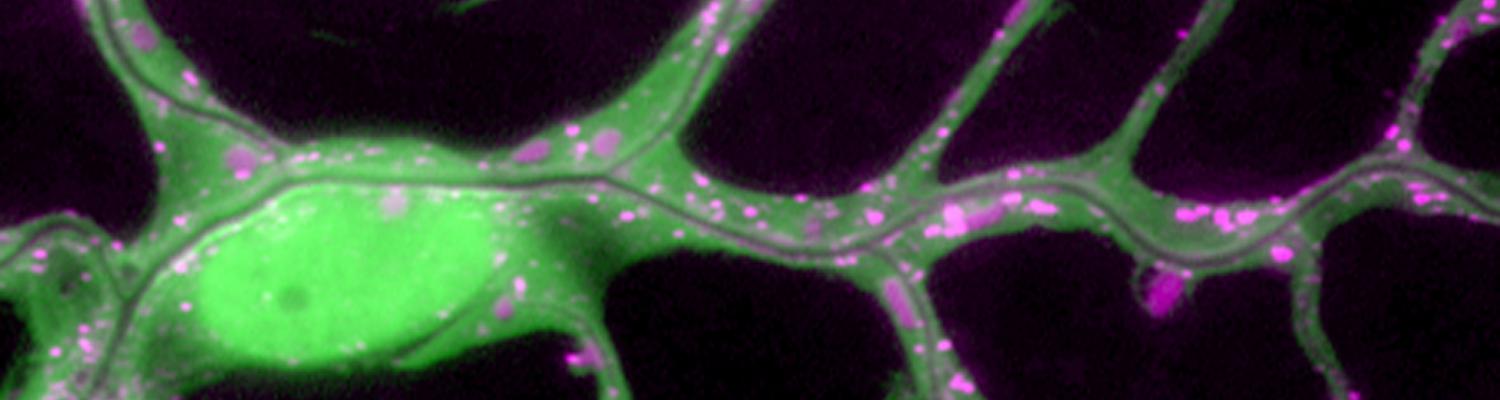
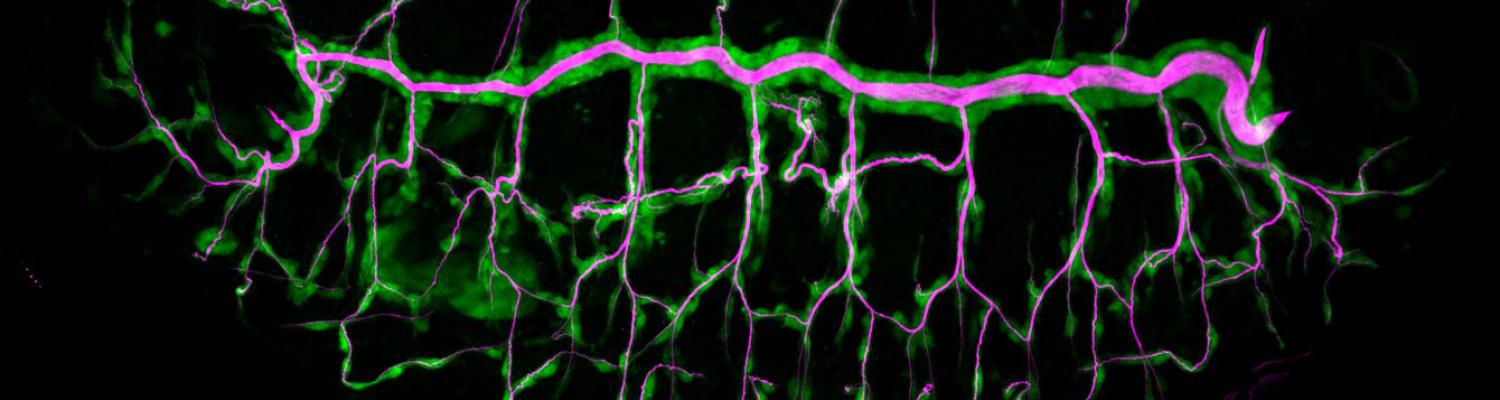
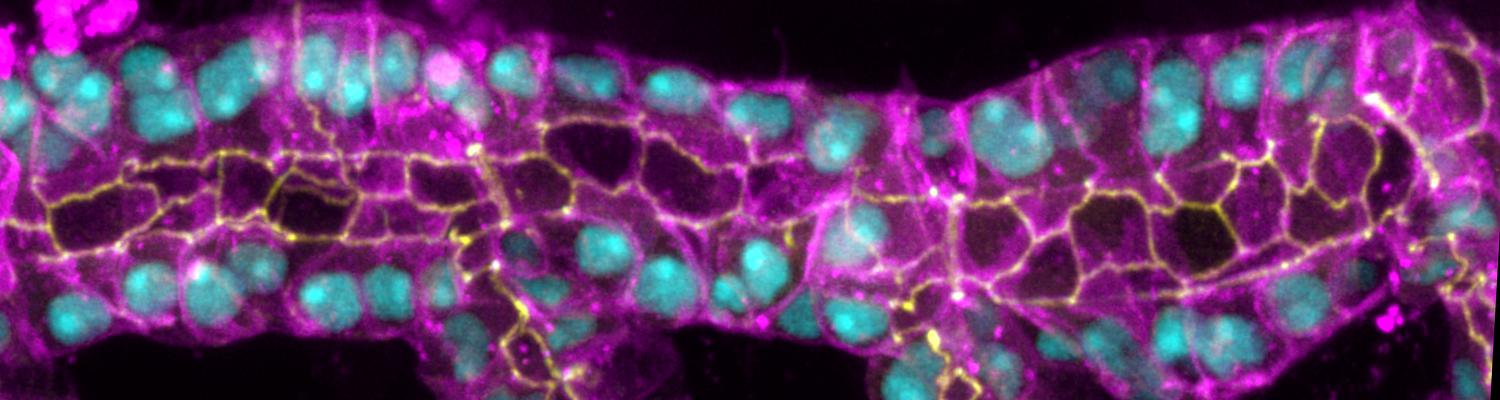
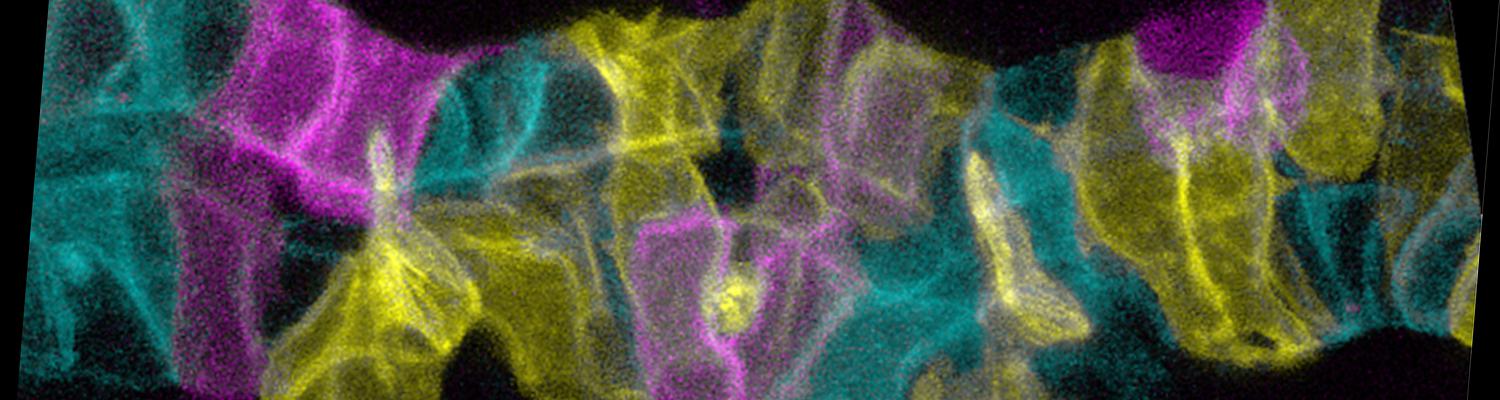
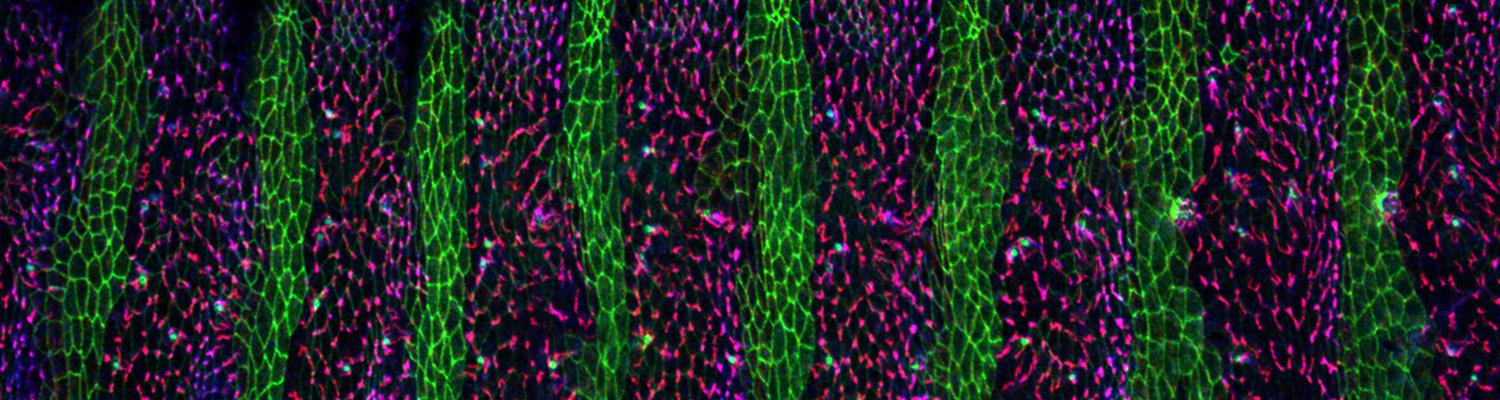
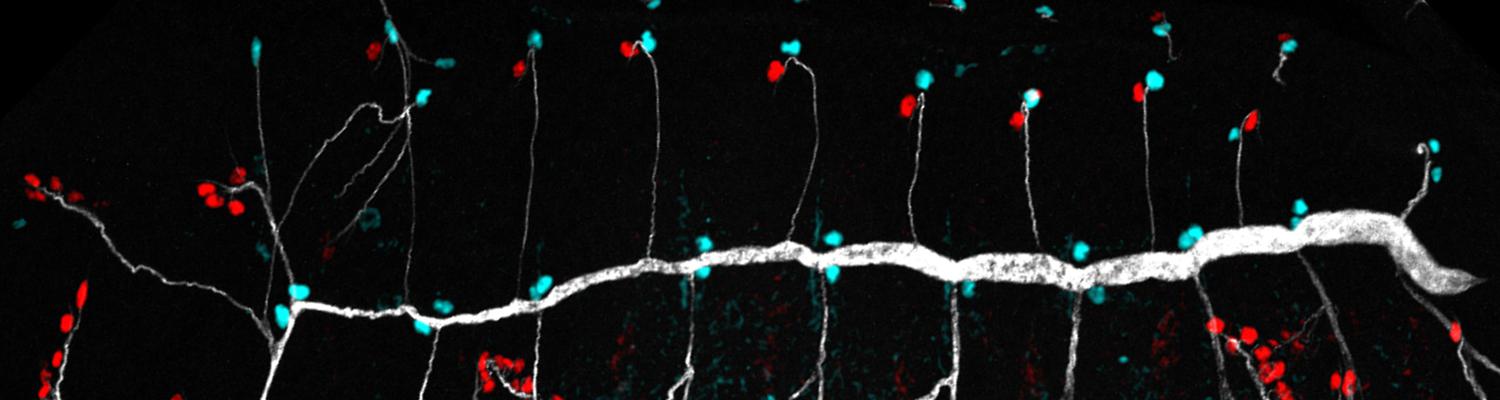
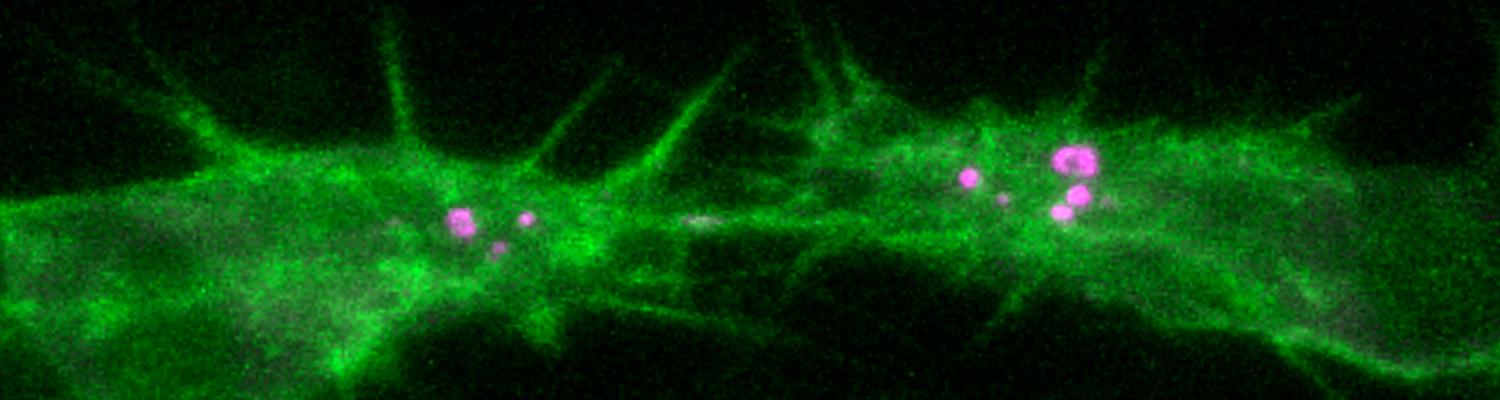
We investigate the assembly and function of epithelial barriers. Tight junctions in vertebrates and septate junctions in invertebrates form diffusion barriers by sealing the paracellular space between plasma membranes of neighboring cells. Junctions between two adjacent cells (bicellular junctions) have been intensely studied and most of their components are known. However, sealing the epithelium at sites of contact between three cells requires specialized tricellular junctions (TCJs). Despite their fundamental role in epithelial biology, TCJs have received little attention and are poorly described in terms of their structure, composition, and the dynamics of their assembly and maintenance. We characterized Anakonda (Aka), a new TCJ protein with an unusual triple-repeat structure, which plays a key role in TCJ assembly and function (Byri et al. 2015).
Formation and Function of Epithelial Tricellular Junctions
Related Publications
By: Byri S, Misra T, Syed ZA, Bätz T, Shah J, Boril L, Glashauser J, Aegerter-Wilmsen T, Matzat T, Moussian B, Uv A, Luschnig S
Developmental Cell | | Volume: 33 | Issue: 5 | 535-48 |Abstract
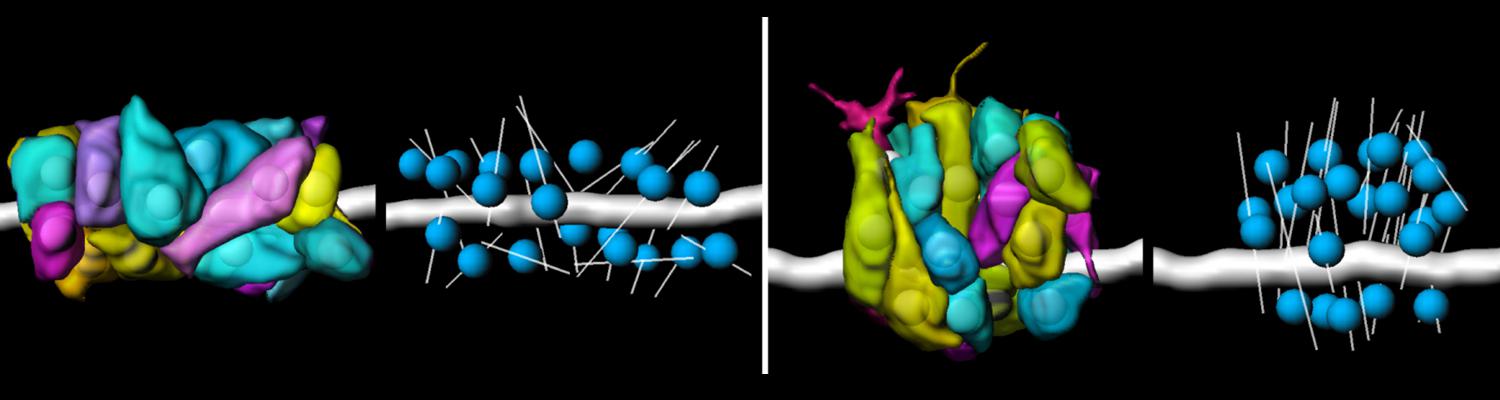
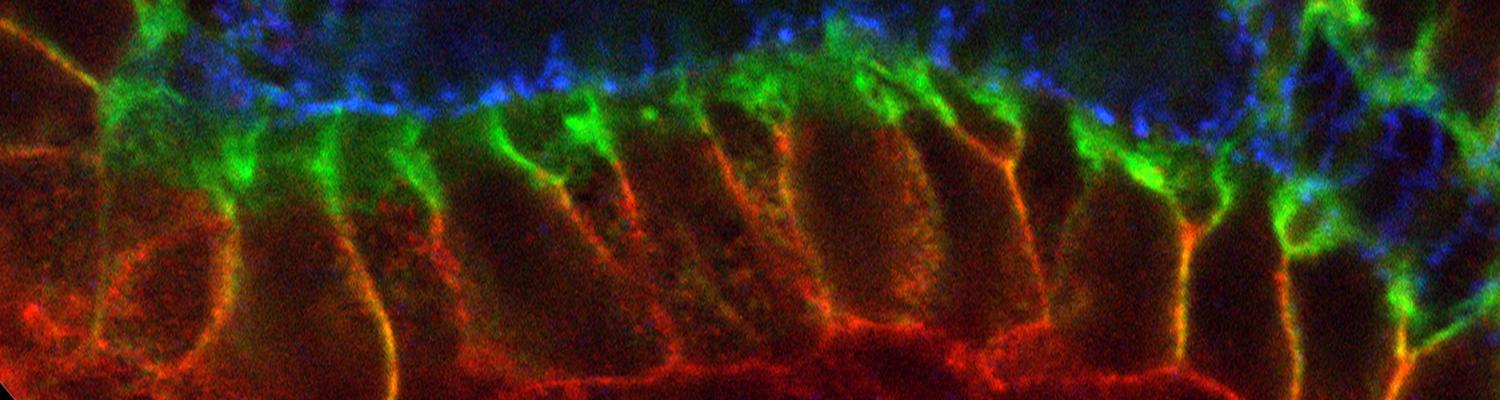
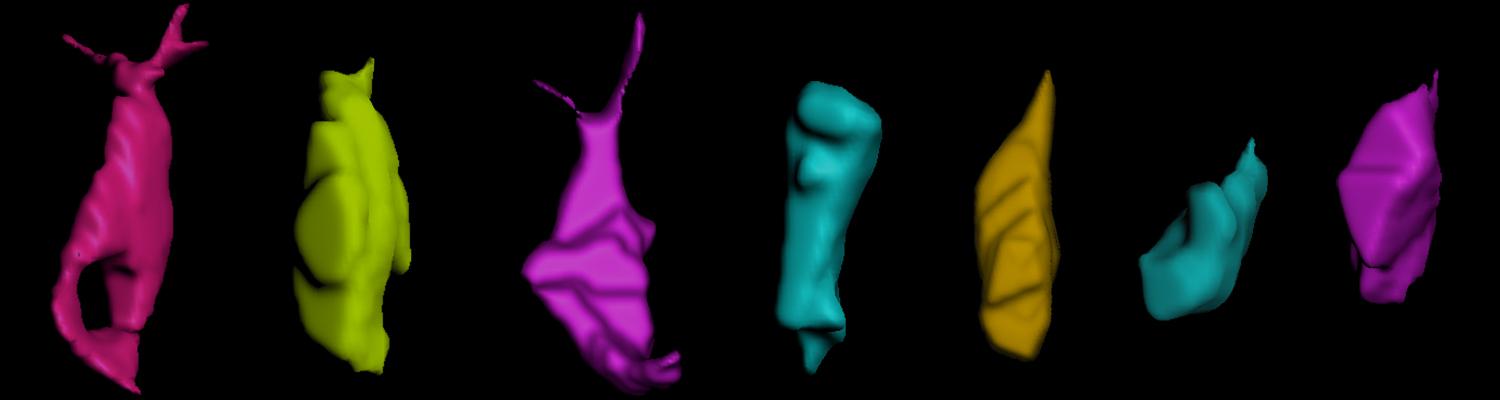
In epithelia, specialized tricellular junctions (TCJs) mediate cell contacts at three-cell vertices. TCJs are fundamental to epithelial biology and disease, but only a few TCJ components are known, and how they assemble at tricellular vertices is not understood. Here we describe a transmembrane protein, Anakonda (Aka), which localizes to TCJs and is essential for the formation of tricellular, but not bicellular, junctions in Drosophila. Loss of Aka causes epithelial barrier defects associated with irregular TCJ structure and geometry, suggesting that Aka organizes cell corners. Aka is necessary and sufficient for accumulation of Gliotactin at TCJs, suggesting that Aka initiates TCJ assembly by recruiting other proteins to tricellular vertices. Aka's extracellular domain has an unusual tripartite repeat structure that may mediate self-assembly, directed by the geometry of tricellular vertices. Conversely, Aka's cytoplasmic tail is dispensable for TCJ localization. Thus, extracellular interactions, rather than TCJ-directed intracellular transport, appear to mediate TCJ assembly.
By: Bätz T, Förster D, Luschnig S
Development | | Volume: 141 | Issue: 4 | 899-908 |Abstract
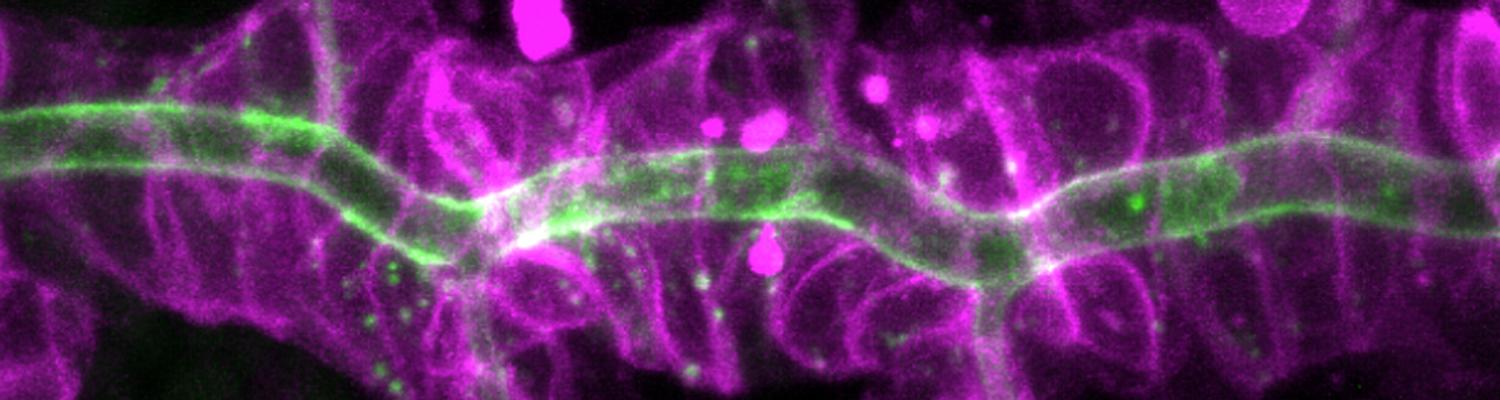
Occluding cell-cell junctions in epithelia form physical barriers that separate different membrane domains, restrict paracellular diffusion and prevent pathogens from spreading across tissues. In invertebrates, these functions are provided by septate junctions (SJs), the functional equivalent of vertebrate tight junctions. How the diverse functions of SJs are integrated and modulated in a multiprotein complex is not clear, and many SJ components are still unknown. Here we report the identification of Macroglobulin complement-related (Mcr), a member of the conserved α-2-macroglobulin (α2M) complement protein family, as a novel SJ-associated protein in Drosophila. Whereas α2M complement proteins are generally known as secreted factors that bind to surfaces of pathogens and target them for phagocytic uptake, Mcr represents an unusual α2M protein with a predicted transmembrane domain. We show that Mcr protein localizes to lateral membranes of epithelial cells, where its distribution overlaps with SJs. Several SJ components are required for the correct localization of Mcr. Conversely, Mcr is required in a cell-autonomous fashion for the correct membrane localization of SJ components, indicating that membrane-bound rather than secreted Mcr isoforms are involved in SJ formation. Finally, we show that loss of Mcr function leads to morphological, ultrastructural and epithelial barrier defects resembling mutants lacking SJ components. Our results, along with previous findings on the role of Mcr in phagocytosis, suggest that Mcr plays dual roles in epithelial barrier formation and innate immunity. Thus, Mcr represents a novel paradigm for investigating functional links between occluding junction formation and pathogen defense mechanisms.